右美沙芬:修订间差异
小无编辑摘要 |
小无编辑摘要 |
||
| (未显示同一用户的12个中间版本) | |||
| 第1行: | 第1行: | ||
{{Drugbox |
|||
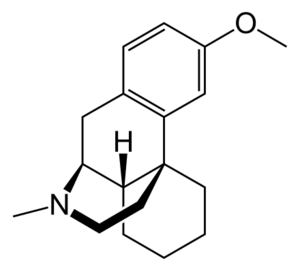
'''右美沙芬'''(英文:[[Dextromethorphan]]),又名右甲吗喃,英文简称DM或DXM,是一种[[处方]]中枢性止咳药,它的氢溴酸盐(Dextromethorphan hydrobromide)常用于药品中,也就是常见的[[氢溴酸右美沙芬]]片或氢溴酸右美沙芬糖浆。主要用于[[干咳]],通过抑制[[延髓]][[咳嗽中枢]]而发挥中枢性镇咳作用,无镇痛作用。 |
|||
| image = Structure_of_dextromethorphan.png |
|||
<!-- 化学数据 --> |
|||
== 注意事项 == |
|||
| C = 18 | H = 25 | N = 1 | O = 1 |
|||
偶有头晕、轻度嗜睡、口干、便秘等副作用,孕妇及痰多病人慎用,妊娠3个月内妇女及有精神病史者禁用。4小时内应不超过120mg。 |
|||
<!-- 识别信息 --> |
|||
== 化学 == |
|||
| IUPAC_name = (4b<i>S</i>,8a<i>R</i>,9<i>S</i>)-3-Methoxy-11-methyl-6,7,8,8a,9,10-hexahydro-5H-9,4b-(epiminoethano)phenanthrene |
|||
右美沙芬的化学式为C<sub>18</sub>H<sub>25</sub>NO,化学结构为: |
|||
| CAS_number = 125-71-3 |
|||
[[File:右美沙芬化学结构.png|thumb|left]] |
|||
| PubChem = 5360696 |
|||
<!-- 药理数据 --> |
|||
| Pharmacology_refs = <ref name="NguyenThomas2016"/> |
|||
| NMDAR_action = antagonist |
|||
| NMDAR_Binding_site = MK-801/PCP |
|||
| NMDAR_Ki = 2.120~8.945 |
|||
| 5HT1A_Ki = >1 |
|||
| 5HT1B_Ki = 1(61%) |
|||
| 5HT1D_Ki = 1(61%) |
|||
| 5HT2A_Ki = >1 |
|||
| ADRA1_Ki = >1 |
|||
| ADRA2_Ki = 1(60%) |
|||
| HRH1_Ki = >1 |
|||
| DRD2_Ki = >1 |
|||
| ERG2_Ki = 0.142~0.652 |
|||
| TMEM97_Ki = 11.06~22.864 |
|||
| SERT_Ki = 0.023~0.04 |
|||
| DAT_Ki = >1 |
|||
| NET_Ki = ≥0.24 |
|||
}} |
|||
'''右美沙芬'''(英文:Dextromethorphan),又名右甲吗喃,英文简称DM或DXM,是中枢性止咳药,它的氢溴酸盐(Dextromethorphan hydrobromide)常用于药品中,也就是常见的氢溴酸右美沙芬片或氢溴酸右美沙芬糖浆。主要用于[[干咳]]而[[湿咳]]应慎用,通过抑制[[延髓]][[咳嗽中枢]]而发挥中枢性镇咳作用。 |
|||
== 药品说明书 == |
|||
右美沙芬片每片15mg,成人口服一次1~2片,一日3~4次。哮喘、痰多、肝肾功能不全患者及孕妇慎用。医疗剂量下可见头晕、头痛、嗜睡、易激动、嗳气、食欲缺乏、便秘、恶心、皮肤过敏等副作用,过量时可见神志不清、幻觉、支气管痉挛、呼吸抑制。<ref>{{cite web |url=https://ypk.familydoctor.com.cn/39956/instructions/ |title=右美沙芬片(氢溴酸右美沙芬片) |language=中文 |accessdate=2024-01-04 }}</ref> |
|||
== 药理学 == |
|||
=== 药效学 === |
|||
{| class="wikitable sortable floatright" style="font-size:small;" |
|||
|+ 右美沙芬及其代谢物结合的受体<ref name="PDSP">{{cite web | title = PDSP K<sub>i</sub> Database | work = Psychoactive Drug Screening Program (PDSP)|author1-link=Bryan Roth | vauthors = Roth BL, Driscol J | publisher = University of North Carolina at Chapel Hill and the United States National Institute of Mental Health | access-date = 14 August 2017 | url = https://pdsp.unc.edu/databases/pdsp.php?knowID=0&kiKey=&receptorDD=&receptor=&speciesDD=&species=&sourcesDD=&source=&hotLigandDD=&hotLigand=&testLigandDD=&testFreeRadio=testFreeRadio&testLigand=dextromethorphan&referenceDD=&reference=&KiGreater=&KiLess=&kiAllRadio=all&doQuery=Submit+Query}}</ref><ref name="NguyenThomas2016">{{cite journal | vauthors = Nguyen L, Thomas KL, Lucke-Wold BP, Cavendish JZ, Crowe MS, Matsumoto RR | title = Dextromethorphan: An update on its utility for neurological and neuropsychiatric disorders | journal = Pharmacology & Therapeutics | volume = 159 | pages = 1–22 | date = March 2016 | pmid = 26826604 | doi = 10.1016/j.pharmthera.2016.01.016 }}</ref><ref name="pmid17689532">{{cite journal | vauthors = Werling LL, Keller A, Frank JG, Nuwayhid SJ | title = A comparison of the binding profiles of dextromethorphan, memantine, fluoxetine and amitriptyline: treatment of involuntary emotional expression disorder | journal = Experimental Neurology | volume = 207 | issue = 2 | pages = 248–257 | date = October 2007 | pmid = 17689532 | doi = 10.1016/j.expneurol.2007.06.013 | s2cid = 38476281 }}</ref><ref name="pmid27139517">{{cite journal | vauthors = Taylor CP, Traynelis SF, Siffert J, Pope LE, Matsumoto RR | title = Pharmacology of dextromethorphan: Relevance to dextromethorphan/quinidine (Nuedexta®) clinical use | journal = Pharmacology & Therapeutics | volume = 164 | pages = 170–182 | date = August 2016 | pmid = 27139517 | doi = 10.1016/j.pharmthera.2016.04.010 | doi-access = free }}</ref> |
|||
|- |
|||
! 位点 !! {{abbr|DXM|右美沙芬}} !! {{abbrlink|DXO|右啡烷}} !! 来源 !! 参考 |
|||
|- |
|||
| [[NMDA受体|{{abbr|NMDAR|N-甲基-D-天冬氨酸受体}}<br/>(MK-801)]] || 2,120–8,945 || 486–906 || 鼠 || <ref name="NguyenThomas2016"/> |
|||
|- |
|||
| [[σ-1受体|σ<sub>1</sub>]] || 142–652 || 118–481 || 老鼠 || |
|||
|- |
|||
| [[σ-2受体|σ<sub>2</sub>]] || 11,060–22,864 || 11,325–15,582 || 鼠 || <ref name="NguyenThomas2016"/> |
|||
|- |
|||
| {{abbrlink|MOR|μ-阿片受体}} || 1,280<br />{{abbr|ND|No data}} || 420<br />>1,000 || 鼠<br/>人类|| <ref name="NguyenThomas2016"/><br /><ref name="pmid7815359">{{cite journal | vauthors = Raynor K, Kong H, Mestek A, Bye LS, Tian M, Liu J, Yu L, Reisine T | display-authors = 6 | title = Characterization of the cloned human mu opioid receptor | journal = The Journal of Pharmacology and Experimental Therapeutics | volume = 272 | issue = 1 | pages = 423–428 | date = January 1995 | pmid = 7815359 }}</ref> |
|||
|- |
|||
| {{abbrlink|DOR|δ-阿片受体}} || 11,500 || 34,700 || 鼠 || <ref name="NguyenThomas2016"/> |
|||
|- |
|||
| {{abbrlink|KOR|κ-阿片受体}} || 7,000 || 5,950 || 鼠 || <ref name="NguyenThomas2016"/> |
|||
|- |
|||
| {{abbrlink|SERT|血清素转运体}} || 23–40 || 401–484 || 鼠 || <ref name="NguyenThomas2016"/> |
|||
|- |
|||
| {{abbrlink|NET|去甲肾上腺素转运体}} || ≥240 || ≥340 || 鼠 || <ref name="NguyenThomas2016"/> |
|||
|- |
|||
| {{abbrlink|DAT|多巴胺转运体}} || >1,000 || >1,000 || 鼠 || <ref name="NguyenThomas2016"/> |
|||
|- |
|||
| [[血清素1A受体|5-HT<sub>1A</sub>]] || >1,000 || >1,000 || 鼠 || <ref name="NguyenThomas2016"/> |
|||
|- |
|||
| [[血清素1B受体|5-HT<sub>1B</sub>]]<sub>/</sub>[[5-HT1D receptor|<sub>1D</sub>]] || 61% at 1 μM || 54% at 1 μM || 鼠 || <ref name="NguyenThomas2016"/> |
|||
|- |
|||
| [[血清素2A受体|5-HT<sub>2A</sub>]] || >1,000 || >1,000 || 鼠 || <ref name="NguyenThomas2016"/> |
|||
|- |
|||
| [[α-1肾上腺素能受体|α<sub>1</sub>]] || >1,000 || >1,000 || 鼠 || <ref name="NguyenThomas2016"/> |
|||
|- |
|||
| [[α-2肾上腺素能受体|α<sub>2</sub>]] || 60% at 1 μM || >1,000 || 鼠 || <ref name="NguyenThomas2016"/> |
|||
|- |
|||
| [[β-肾上腺素能受体|β]] || >1,000 || 35% at 1 μM || 鼠 || <ref name="NguyenThomas2016"/> |
|||
|- |
|||
| [[多巴胺D2受体|D<sub>2</sub>]] || >1,000 || >1,000 || 鼠 || <ref name="NguyenThomas2016"/> |
|||
|- |
|||
| [[组胺H1受体|H<sub>1</sub>]] || >1,000 || 95% at 1 μM || 鼠 || <ref name="NguyenThomas2016"/> |
|||
|- |
|||
| {{abbrlink|mAChRs|毒蕈碱乙酰胆碱受体}} || >1,000 || 100% at 1 μM || 鼠 || <ref name="NguyenThomas2016"/> |
|||
|- |
|||
| {{abbrlink|nAChRs|烟碱乙酰胆碱受体}} || 700–8,900<br />([[IC50|IC<sub>50</sub>]]) || 1,300–29,600<br />(IC<sub>50</sub>) || 鼠 || <ref name="NguyenThomas2016"/> |
|||
|- |
|||
| {{abbrlink|VDSCs|电压依赖性钠通道}} || >50,000 (IC<sub>50</sub>) || {{abbr|ND|No data}} || 鼠 || <ref name="pmid17346698">{{cite journal | vauthors = Lee JH, Shin EJ, Jeong SM, Lee BH, Yoon IS, Lee JH, Choi SH, Kim YH, Pyo MK, Lee SM, Chae JS, Rhim H, Oh JW, Kim HC, Nah SY | display-authors = 6 | title = Effects of dextrorotatory morphinans on brain Na+ channels expressed in Xenopus oocytes | journal = European Journal of Pharmacology | volume = 564 | issue = 1–3 | pages = 7–17 | date = June 2007 | pmid = 17346698 | doi = 10.1016/j.ejphar.2007.01.088 }}</ref><ref name="pmid23139844">{{cite journal | vauthors = Gao XF, Yao JJ, He YL, Hu C, Mei YA | title = Sigma-1 receptor agonists directly inhibit Nav1.2/1.4 channels | journal = PLOS ONE | volume = 7 | issue = 11 | pages = e49384 | year = 2012 | pmid = 23139844 | pmc = 3489664 | doi = 10.1371/journal.pone.0049384 | doi-access = free | bibcode = 2012PLoSO...749384G }}</ref> |
|||
|- class="sortbottom" |
|||
| colspan="5" style="width: 1px;" | 除非另有说明,数值均为 K<sub>i</sub>(nM)。该值越小则药物与该位点的结合越强。 |
|||
|} |
|||
右美沙芬通过[[MK-801]]/[[PCP]]位点非竞争性拮抗[[NMDA受体]]。<ref name="pmid24648790">{{cite journal | vauthors = Burns JM, Boyer EW | title = Antitussives and substance abuse | journal = Substance Abuse and Rehabilitation | volume = 4 | pages = 75–82 | year = 2013 | pmid = 24648790 | pmc = 3931656 | doi = 10.2147/SAR.S36761 | doi-access = free }}</ref>它还是血清素和去甲肾上腺素转运体抑制剂(SNRI);是σ-1受体的激动剂;是[[烟碱乙酰胆碱受体]]的[[负变构调节剂]];右美沙芬还是[[血清素]][[5HT1B|1B]]/[[5HT1D|1D]]、[[组胺H1]]、α2-肾上腺素能和[[毒蕈碱乙酰胆碱受体]]的[[配体]]。<ref name="NguyenThomas2016"/><ref name="pmid24648790" /> |
|||
右美沙芬的代谢物之一[[右啡烷]]作为比右美沙芬更强的NMDA拮抗剂,通常比右美沙芬更造成[[解离]]。<ref name="pmid10064839">{{cite journal | vauthors = Chou YC, Liao JF, Chang WY, Lin MF, Chen CF | title = Binding of dimemorfan to sigma-1 receptor and its anticonvulsant and locomotor effects in mice, compared with dextromethorphan and dextrorphan | journal = Brain Research | volume = 821 | issue = 2 | pages = 516–519 | date = March 1999 | pmid = 10064839 | doi = 10.1016/S0006-8993(99)01125-7 | s2cid = 22762264 }}</ref>而右美沙芬的另一种主要代谢物(+)-3-甲氧基吗啡喃的作用尚不完全明确。<ref>{{cite journal | vauthors = Schmider J, Greenblatt DJ, Fogelman SM, von Moltke LL, Shader RI | title = Metabolism of dextromethorphan in vitro: involvement of cytochromes P450 2D6 and 3A3/4, with a possible role of 2E1 | journal = Biopharmaceutics & Drug Disposition | volume = 18 | issue = 3 | pages = 227–240 | date = April 1997 | pmid = 9113345 | doi = 10.1002/(SICI)1099-081X(199704)18:3<227::AID-BDD18>3.0.CO;2-L | s2cid = 5638973 }}</ref> |
|||
=== 药代动力学 === |
|||
右美沙芬在口服后大约15~30分钟内开始起效,在2~3小时达到[[血药峰值]],它的生物半衰期在代谢能力强的人身上大约为2~4小时,而代谢能力弱的人有可能到达24小时,大部分通过尿液排出。<ref name = MSR>{{cite web|title=Balminil DM, Benylin DM (dextromethorphan) dosing, indications, interactions, adverse effects, and more|work=Medscape Reference|publisher=WebMD|accessdate=2024-02-11|url=http://reference.medscape.com/drug/balminil-dm-benylin-dm-dextromethorphan-343401#showall|archive-date=2019-03-31|archive-url=https://web.archive.org/web/20190331154154/https://reference.medscape.com/drug/balminil-dm-benylin-dm-dextromethorphan-343401#showall|dead-url=no}}</ref> |
|||
右美沙芬口服时经过首过效应让部分药物通过O-去甲基化代谢成右美沙芬的活性代谢产物右啡烷,同时通过N-去甲基化生成3-甲氧基吗啡喃(MEM),<ref name="DXMdualprobe">{{cite journal | vauthors = Yu A, Haining RL | title = Comparative contribution to dextromethorphan metabolism by cytochrome P450 isoforms in vitro: can dextromethorphan be used as a dual probe for both CTP2D6 and CYP3A activities? | journal = Drug Metabolism and Disposition | volume = 29 | issue = 11 | pages = 1514–1520 | date = November 2001 | pmid = 11602530 | url = http://dmd.aspetjournals.org/cgi/pmidlookup?view=long&pmid=11602530 | access-date = 2015-04-26 | archive-date = 2020-03-12 | archive-url = https://web.archive.org/web/20200312134718/http://dmd.aspetjournals.org/content/29/11/1514.long | url-status = dead }}</ref><ref name="nhtsa">{{cite web|url=http://www.nhtsa.dot.gov/PEOPLE/injury/research/job185drugs/dextromethorphan.htm|title=Dextromethorphan|work=National Highway Traffic Safety Administration (NHTSA)|archive-url=https://web.archive.org/web/20080801054711/http://www.nhtsa.dot.gov/PEOPLE/injury/research/job185drugs/dextromethorphan.htm|archive-date=2008-08-01}}</ref>并与葡萄糖醛酸和硫酸根离子部分结合。右美沙芬最主要的代谢产物是右啡烷,其中80%的右啡烷是由CYP2D6产生的,在CYP2D6酶活性较差的人身上可能会使AUC增加150倍、中位半衰期延长至6~8倍。<ref name="pmid8841152">{{cite journal | vauthors = Capon DA, Bochner F, Kerry N, Mikus G, Danz C, Somogyi AA | title = The influence of CYP2D6 polymorphism and quinidine on the disposition and antitussive effect of dextromethorphan in humans | journal = Clinical Pharmacology and Therapeutics | volume = 60 | issue = 3 | pages = 295–307 | date = September 1996 | pmid = 8841152 | doi = 10.1016/S0009-9236(96)90056-9 | s2cid = 10147669 }}</ref>右美沙芬的代谢产物之一3-甲氧基吗啡喃约90%都是通过CYP3A4的N-去甲基化产生的。<ref name="pmid8841152"></ref> |
|||
== 药物滥用 == |
|||
右美沙芬时常被滥用,其中属未成年中较为流行。<ref>{{cite web |url=https://new.qq.com/rain/a/20221229A04TJH00 |title=右美沙芬滥用者的隐秘世界 |publisher=苏子涵 、李沁桦、刘汨 |date=2022-12-29 |accessdate=2024-02-11 }}</ref>由于其被滥用的潜质,中国国家药监局在2021年12月16日将右美沙芬口服单方制从[[非处方药]]剂转换为[[处方药]]并随后实行[[网络禁售]]。<ref name="Rx-upgrade">{{cite news |title=国家药监局关于氢溴酸右美沙芬口服单方制剂转换为处方药的公告(2021年第151号) |url=https://www.nmpa.gov.cn/directory/web/nmpa/yaopin/ypggtg/20211227162159192.html |accessdate=2024-02-11 |publisher=国家药品监督管理局 |date=2021-12-16 |archive-date=2022-02-15 |archive-url=https://web.archive.org/web/20220215013414/https://www.nmpa.gov.cn/directory/web/nmpa/xxgk/ggtg/qtggtg/20211227162159192.html |dead-url=no }}</ref><ref>{{cite web |url=https://www.nmpa.gov.cn/yaopin/ypggtg/20221130200847133.html |title=国家药监局关于发布药品网络销售禁止清单(第一版)的公告(2022年 第111号) |publisher=国家药品监督管理局 |date=2022-11-30 |accessdate=2024-02-11 }}</ref>并在2024年5月将其进一步加入精神药品目录(7月起实行)。 |
|||
== 法律地位 == |
|||
=== 中国 === |
|||
中国大陆在2021年将右美沙芬从非处方药改为处方药,<ref name="Rx-upgrade"></ref>随后又在2024年列入[[精神药品品种目]]第二类。<ref>{{cite web |url=https://www.nmpa.gov.cn/xxgk/ggtg/ypggtg/ypqtggtg/20240507084000186.html |title=国家药监局 公安部 国家卫生健康委关于调整精神药品目录的公告(2024年第54号) |date=2024-05-07 |accessdate=2024-05-07 }}</ref> |
|||
== 参考文献 == |
|||
{{Reflist}} |
|||
{{中华人民共和国管制药品}} |
|||
2024年9月25日 (三) 07:22的最新版本
 | |
| 化学数据 | |
|---|---|
| 化学式 | C18H25NO |
| 摩尔质量 | 271.40 g·mol−1 |
| 识别信息 | |
| IUPAC名称 | (4bS,8aR,9S)-3-Methoxy-11-methyl-6,7,8,8a,9,10-hexahydro-5H-9,4b-(epiminoethano)phenanthrene |
| CAS号 | |
| PubChem CID | |
| 药理数据[1] | |
| 5HT1A | |
| 5HT1B | |
| 5HT1D | |
| 5HT2A | |
| HRH1 | |
| NMDAR(↓) | |
| DRD2 | |
| SERT | |
| DAT | |
| NET | |
| ADRA1 | |
| ADRA2 | |
| ERG2 | |
| TMEM97 | |
右美沙芬(英文:Dextromethorphan),又名右甲吗喃,英文简称DM或DXM,是中枢性止咳药,它的氢溴酸盐(Dextromethorphan hydrobromide)常用于药品中,也就是常见的氢溴酸右美沙芬片或氢溴酸右美沙芬糖浆。主要用于干咳而湿咳应慎用,通过抑制延髓咳嗽中枢而发挥中枢性镇咳作用。
药品说明书
右美沙芬片每片15mg,成人口服一次1~2片,一日3~4次。哮喘、痰多、肝肾功能不全患者及孕妇慎用。医疗剂量下可见头晕、头痛、嗜睡、易激动、嗳气、食欲缺乏、便秘、恶心、皮肤过敏等副作用,过量时可见神志不清、幻觉、支气管痉挛、呼吸抑制。[2]
药理学
药效学
| 位点 | DXM | DXO | 来源 | 参考 |
|---|---|---|---|---|
| NMDAR (MK-801) |
2,120–8,945 | 486–906 | 鼠 | [1] |
| σ1 | 142–652 | 118–481 | 老鼠 | |
| σ2 | 11,060–22,864 | 11,325–15,582 | 鼠 | [1] |
| MOR | 1,280 ND |
420 >1,000 |
鼠 人类 |
[1] [6] |
| DOR | 11,500 | 34,700 | 鼠 | [1] |
| KOR | 7,000 | 5,950 | 鼠 | [1] |
| SERT | 23–40 | 401–484 | 鼠 | [1] |
| NET | ≥240 | ≥340 | 鼠 | [1] |
| DAT | >1,000 | >1,000 | 鼠 | [1] |
| 5-HT1A | >1,000 | >1,000 | 鼠 | [1] |
| 5-HT1B/1D | 61% at 1 μM | 54% at 1 μM | 鼠 | [1] |
| 5-HT2A | >1,000 | >1,000 | 鼠 | [1] |
| α1 | >1,000 | >1,000 | 鼠 | [1] |
| α2 | 60% at 1 μM | >1,000 | 鼠 | [1] |
| β | >1,000 | 35% at 1 μM | 鼠 | [1] |
| D2 | >1,000 | >1,000 | 鼠 | [1] |
| H1 | >1,000 | 95% at 1 μM | 鼠 | [1] |
| mAChRs | >1,000 | 100% at 1 μM | 鼠 | [1] |
| nAChRs | 700–8,900 (IC50) |
1,300–29,600 (IC50) |
鼠 | [1] |
| VDSCs | >50,000 (IC50) | ND | 鼠 | [7][8] |
| 除非另有说明,数值均为 Ki(nM)。该值越小则药物与该位点的结合越强。 | ||||
右美沙芬通过MK-801/PCP位点非竞争性拮抗NMDA受体。[9]它还是血清素和去甲肾上腺素转运体抑制剂(SNRI);是σ-1受体的激动剂;是烟碱乙酰胆碱受体的负变构调节剂;右美沙芬还是血清素1B/1D、组胺H1、α2-肾上腺素能和毒蕈碱乙酰胆碱受体的配体。[1][9]
右美沙芬的代谢物之一右啡烷作为比右美沙芬更强的NMDA拮抗剂,通常比右美沙芬更造成解离。[10]而右美沙芬的另一种主要代谢物(+)-3-甲氧基吗啡喃的作用尚不完全明确。[11]
药代动力学
右美沙芬在口服后大约15~30分钟内开始起效,在2~3小时达到血药峰值,它的生物半衰期在代谢能力强的人身上大约为2~4小时,而代谢能力弱的人有可能到达24小时,大部分通过尿液排出。[12]
右美沙芬口服时经过首过效应让部分药物通过O-去甲基化代谢成右美沙芬的活性代谢产物右啡烷,同时通过N-去甲基化生成3-甲氧基吗啡喃(MEM),[13][14]并与葡萄糖醛酸和硫酸根离子部分结合。右美沙芬最主要的代谢产物是右啡烷,其中80%的右啡烷是由CYP2D6产生的,在CYP2D6酶活性较差的人身上可能会使AUC增加150倍、中位半衰期延长至6~8倍。[15]右美沙芬的代谢产物之一3-甲氧基吗啡喃约90%都是通过CYP3A4的N-去甲基化产生的。[15]
药物滥用
右美沙芬时常被滥用,其中属未成年中较为流行。[16]由于其被滥用的潜质,中国国家药监局在2021年12月16日将右美沙芬口服单方制从非处方药剂转换为处方药并随后实行网络禁售。[17][18]并在2024年5月将其进一步加入精神药品目录(7月起实行)。
法律地位
中国
中国大陆在2021年将右美沙芬从非处方药改为处方药,[17]随后又在2024年列入精神药品品种目第二类。[19]
参考文献
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 Nguyen L, Thomas KL, Lucke-Wold BP, Cavendish JZ, Crowe MS, Matsumoto RR (March 2016). "Dextromethorphan: An update on its utility for neurological and neuropsychiatric disorders". Pharmacology & Therapeutics. 159: 1–22. doi:10.1016/j.pharmthera.2016.01.016. PMID 26826604.
- ↑ "右美沙芬片(氢溴酸右美沙芬片)" (in 中文). Retrieved 2024-01-04.
- ↑ Roth BL, Driscol J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 14 August 2017.
- ↑ Werling LL, Keller A, Frank JG, Nuwayhid SJ (October 2007). "A comparison of the binding profiles of dextromethorphan, memantine, fluoxetine and amitriptyline: treatment of involuntary emotional expression disorder". Experimental Neurology. 207 (2): 248–257. doi:10.1016/j.expneurol.2007.06.013. PMID 17689532. S2CID 38476281.
- ↑ Taylor CP, Traynelis SF, Siffert J, Pope LE, Matsumoto RR (August 2016). "Pharmacology of dextromethorphan: Relevance to dextromethorphan/quinidine (Nuedexta®) clinical use". Pharmacology & Therapeutics. 164: 170–182. doi:10.1016/j.pharmthera.2016.04.010. PMID 27139517.
- ↑ Raynor K, Kong H, Mestek A, Bye LS, Tian M, Liu J, et al. (January 1995). "Characterization of the cloned human mu opioid receptor". The Journal of Pharmacology and Experimental Therapeutics. 272 (1): 423–428. PMID 7815359.
- ↑ Lee JH, Shin EJ, Jeong SM, Lee BH, Yoon IS, Lee JH, et al. (June 2007). "Effects of dextrorotatory morphinans on brain Na+ channels expressed in Xenopus oocytes". European Journal of Pharmacology. 564 (1–3): 7–17. doi:10.1016/j.ejphar.2007.01.088. PMID 17346698.
- ↑ Gao XF, Yao JJ, He YL, Hu C, Mei YA (2012). "Sigma-1 receptor agonists directly inhibit Nav1.2/1.4 channels". PLOS ONE. 7 (11): e49384. Bibcode:2012PLoSO...749384G. doi:10.1371/journal.pone.0049384. PMC 3489664. PMID 23139844.
- ↑ 9.0 9.1 Burns JM, Boyer EW (2013). "Antitussives and substance abuse". Substance Abuse and Rehabilitation. 4: 75–82. doi:10.2147/SAR.S36761. PMC 3931656. PMID 24648790.
- ↑ Chou YC, Liao JF, Chang WY, Lin MF, Chen CF (March 1999). "Binding of dimemorfan to sigma-1 receptor and its anticonvulsant and locomotor effects in mice, compared with dextromethorphan and dextrorphan". Brain Research. 821 (2): 516–519. doi:10.1016/S0006-8993(99)01125-7. PMID 10064839. S2CID 22762264.
- ↑ Schmider J, Greenblatt DJ, Fogelman SM, von Moltke LL, Shader RI (April 1997). "Metabolism of dextromethorphan in vitro: involvement of cytochromes P450 2D6 and 3A3/4, with a possible role of 2E1". Biopharmaceutics & Drug Disposition. 18 (3): 227–240. doi:10.1002/(SICI)1099-081X(199704)18:3<227::AID-BDD18>3.0.CO;2-L. PMID 9113345. S2CID 5638973.
- ↑ "Balminil DM, Benylin DM (dextromethorphan) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on 2019-03-31. Retrieved 2024-02-11.
{{cite web}}: Unknown parameter|dead-url=ignored (|url-status=suggested) (help) - ↑ Yu A, Haining RL (November 2001). "Comparative contribution to dextromethorphan metabolism by cytochrome P450 isoforms in vitro: can dextromethorphan be used as a dual probe for both CTP2D6 and CYP3A activities?". Drug Metabolism and Disposition. 29 (11): 1514–1520. PMID 11602530. Archived from the original on 2020-03-12. Retrieved 2015-04-26.
- ↑ "Dextromethorphan". National Highway Traffic Safety Administration (NHTSA). Archived from the original on 2008-08-01.
- ↑ 15.0 15.1 Capon DA, Bochner F, Kerry N, Mikus G, Danz C, Somogyi AA (September 1996). "The influence of CYP2D6 polymorphism and quinidine on the disposition and antitussive effect of dextromethorphan in humans". Clinical Pharmacology and Therapeutics. 60 (3): 295–307. doi:10.1016/S0009-9236(96)90056-9. PMID 8841152. S2CID 10147669.
- ↑ "右美沙芬滥用者的隐秘世界". 苏子涵 、李沁桦、刘汨. 2022-12-29. Retrieved 2024-02-11.
- ↑ 17.0 17.1 "国家药监局关于氢溴酸右美沙芬口服单方制剂转换为处方药的公告(2021年第151号)". 国家药品监督管理局. 2021-12-16. Archived from the original on 2022-02-15. Retrieved 2024-02-11.
{{cite news}}: Unknown parameter|dead-url=ignored (|url-status=suggested) (help) - ↑ "国家药监局关于发布药品网络销售禁止清单(第一版)的公告(2022年 第111号)". 国家药品监督管理局. 2022-11-30. Retrieved 2024-02-11.
- ↑ "国家药监局 公安部 国家卫生健康委关于调整精神药品目录的公告(2024年第54号)". 2024-05-07. Retrieved 2024-05-07.