唑吡坦:修订间差异
来自Wikipedia的wikipedia:唑吡坦 |
小无编辑摘要 |
||
| (未显示2个用户的6个中间版本) | |||
| 第1行: | 第1行: | ||
{{drugbox |
{{drugbox |
||
| IUPAC_name = |
| IUPAC_name = <i>N</i>,<i>N<i>,6-三甲基-2-(4-甲基苯基)-咪唑并(1,2-<i>a</i>)吡啶-3-乙酰胺 |
||
| image = Zolpidem structure simple.svg |
| image = Zolpidem structure simple.svg |
||
| image2 = Zolpidem3D.png |
| image2 = Zolpidem3D.png |
||
| 第25行: | 第25行: | ||
}} |
}} |
||
'''唑吡坦'''(英语:''Zolpidem'')是一种[[Z-drugs]][[安眠药]],約在口服三十分 |
'''唑吡坦'''(英语:''Zolpidem'')是一种[[Z-drugs]][[安眠药]],約在口服三十分钟之内起效,药效维持两至三小时。市场上常见的药名包括、'''Adormix'''、'''Ambien'''、'''Ambien CR'''、'''Edluar'''、'''Damixan'''、'''Ivedal'''、'''Nytamel'''、'''Stilnoct'''、'''Stilnox'''、'''Sucedal'''、'''Zoldem'''、'''Zolnod'''及'''Zolpihexal'''等,<ref>{{cite web |author=Ambien.com |year=2004 |title=AMBIEN Prescribing Information |work=Information About a Short-term Treatment for Insomnia - Ambien.com Home Page for Health-care Professionals |publisher=Sanofi-Synthelabo Inc. New York, NY 10016 |url=http://www.ambien.com/hcp/index.asp |accessdate=2005-06-27 |archive-url=https://web.archive.org/web/20050627005720/http://www.ambien.com/hcp/index.asp |archive-date=2005-06-27 |dead-url=yes }}</ref><ref>[http://www.sanofi-aventis.com.au/products/aus_cmi_stilnox.pdf STILNOX (zolpidem tartrate) PRODUCT INFORMATION] {{Wayback|url=http://www.sanofi-aventis.com.au/products/aus_cmi_stilnox.pdf |date=20090913174214 }} Sanofi-Synthelabo Australia Pty Limited. April 15, 2004</ref><ref>{{cite web |url=http://en.sanofi-aventis.com/group/products/p_group_products_nervous_stilnox.asp?ComponentID=1267&SourcePageID=1207#3 |title=sanofi-aventis : Drugs and Products - CNS - Stilnox/Ambien/Myslee |accessdate=2006-11-22 |date=2006-11-07 |archive-url=https://web.archive.org/web/20070927194429/http://en.sanofi-aventis.com/group/products/p_group_products_nervous_stilnox.asp?ComponentID=1267&SourcePageID=1207#3 |archive-date=2007-09-27 |dead-url=yes }}</ref><ref>{{cite web |url=http://www.non-benzodiazepines.org.uk/benzodiazepine-names.html#Zolpidem |title=Benzodiazepine Names |accessdate= |deadurl=yes |archiveurl=https://web.archive.org/web/20081208054743/http://www.non-benzodiazepines.org.uk/benzodiazepine-names.html#Zolpidem |archivedate=2008-12-08 }}</ref>中国大陆商品名为'''思诺思'''(''Stilnox''),台湾則有'''佐沛眠'''(''Zolpium'')、'''使蒂诺斯'''等名称。 |
||
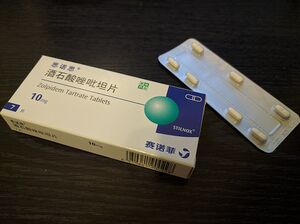
[[File:Stilnox sales in China.jpg|thumb|在中国大陆销售的酒石酸唑吡坦片(思诺思®)]] |
[[File:Stilnox sales in China.jpg|thumb|在中国大陆销售的酒石酸唑吡坦片(思诺思®)]] |
||
==药理学== |
==药理学== |
||
唑吡坦是[[GABAA受体|GABA<sub>A</sub>受体]]的[[别构调节|正别构调节剂]],它选择性结合[[GABAA受体|GABA<sub>A</sub>受体]]的{{External_demand|wikipedia|Gamma-aminobutyric acid receptor subunit alpha-1|γ-氨基丁酸亚基α1|α1}}[[亚基]],因此它具有较强的[[催眠]]和较弱的[[抗焦虑]]、[[肌肉松弛]]和[[抗惊厥]]特性。<ref name="costahalflife">{{cite journal | vauthors = Salvà P, Costa J | title = Clinical pharmacokinetics and pharmacodynamics of zolpidem. Therapeutic implications | journal = Clinical Pharmacokinetics | volume = 29 | issue = 3 | pages = 142–153 | date = September 1995 | pmid = 8521677 | doi = 10.2165/00003088-199529030-00002 | s2cid = 23391285 }}</ref>与[[地西泮]]不同,唑吡坦可以与αβGABAA受体结合,它被证明与α1-α1界面结合。<ref name="pmid27346730">{{cite journal | vauthors = Che Has AT, Absalom N, van Nieuwenhuijzen PS, Clarkson AN, Ahring PK, Chebib M | title = Zolpidem is a potent stoichiometry-selective modulator of α1β3 GABAA receptors: evidence of a novel benzodiazepine site in the α1-α1 interface | journal = Scientific Reports | volume = 6 | pages = 28674 | date = June 2016 | pmid = 27346730 | pmc = 4921915 | doi = 10.1038/srep28674 | bibcode = 2016NatSR...628674C }}</ref>唑吡坦对{{External_demand|wikipedia|Gamma-aminobutyric acid receptor subunit alpha-2|γ-氨基丁酸亚基α2|α2}}-和{{External_demand|wikipedia|Gamma-aminobutyric acid receptor subunit alpha-3|γ-氨基丁酸亚基α3|α3}}-亚基的亲和力比对α<sub>1</sub>低10倍,且对含{{External_demand|wikipedia| |
唑吡坦是[[GABAA受体|GABA<sub>A</sub>受体]]的[[别构调节|正别构调节剂]],它选择性结合[[GABAA受体|GABA<sub>A</sub>受体]]的{{External_demand|wikipedia|Gamma-aminobutyric acid receptor subunit alpha-1|γ-氨基丁酸受体亚基α1|α1}}[[亚基]],因此它具有较强的[[催眠]]和较弱的[[抗焦虑]]、[[肌肉松弛]]和[[抗惊厥]]特性。<ref name="costahalflife">{{cite journal | vauthors = Salvà P, Costa J | title = Clinical pharmacokinetics and pharmacodynamics of zolpidem. Therapeutic implications | journal = Clinical Pharmacokinetics | volume = 29 | issue = 3 | pages = 142–153 | date = September 1995 | pmid = 8521677 | doi = 10.2165/00003088-199529030-00002 | s2cid = 23391285 }}</ref>与[[地西泮]]不同,唑吡坦可以与αβGABAA受体结合,它被证明与α1-α1界面结合。<ref name="pmid27346730">{{cite journal | vauthors = Che Has AT, Absalom N, van Nieuwenhuijzen PS, Clarkson AN, Ahring PK, Chebib M | title = Zolpidem is a potent stoichiometry-selective modulator of α1β3 GABAA receptors: evidence of a novel benzodiazepine site in the α1-α1 interface | journal = Scientific Reports | volume = 6 | pages = 28674 | date = June 2016 | pmid = 27346730 | pmc = 4921915 | doi = 10.1038/srep28674 | bibcode = 2016NatSR...628674C }}</ref>唑吡坦对{{External_demand|wikipedia|Gamma-aminobutyric acid receptor subunit alpha-2|γ-氨基丁酸受体亚基α2|α2}}-和{{External_demand|wikipedia|Gamma-aminobutyric acid receptor subunit alpha-3|γ-氨基丁酸受体亚基α3|α3}}-亚基的亲和力比对α<sub>1</sub>低10倍,且对含{{External_demand|wikipedia|GABRA5|γ-氨基丁酸受体亚基α5|α5}}亚基的受体没有表现出明显的亲和力。<ref name="pmid2157817">{{cite journal | vauthors = Pritchett DB, Seeburg PH | title = Gamma-aminobutyric acidA receptor alpha 5-subunit creates novel type II benzodiazepine receptor pharmacology | journal = Journal of Neurochemistry | volume = 54 | issue = 5 | pages = 1802–4 | date = May 1990 | pmid = 2157817 | doi = 10.1111/j.1471-4159.1990.tb01237.x | s2cid = 86674799 }}</ref><ref name="pmid11306694">{{cite journal | vauthors = Smith AJ, Alder L, Silk J, Adkins C, Fletcher AE, Scales T, Kerby J, Marshall G, Wafford KA, McKernan RM, Atack JR | title = Effect of alpha subunit on allosteric modulation of ion channel function in stably expressed human recombinant gamma-aminobutyric acid(A) receptors determined using (36)Cl ion flux | journal = Molecular Pharmacology | volume = 59 | issue = 5 | pages = 1108–18 | date = May 2001 | pmid = 11306694 | doi = 10.1124/mol.59.5.1108| s2cid = 86156878 }}</ref>含α1亚基的苯二氮卓位点(旧称ω<sub>1</sub>)主要存在于大脑中,而含α2亚基的苯二氮卓位点(旧称ω<sub>2</sub>)主要存在于脊柱中,因此唑吡坦更易于与位于大脑而不是脊柱中的GABA<sub>A</sub>受体结合。<ref>{{cite journal | vauthors = Rowlett JK, Woolverton WL | title = Assessment of benzodiazepine receptor heterogeneity in vivo: apparent pA2 and pKB analyses from behavioral studies | journal = Psychopharmacology | volume = 128 | issue = 1 | pages = 1–16 | date = November 1996 | pmid = 8944400 | doi = 10.1007/s002130050103 | s2cid = 25654504 | url = http://link.springer.de/link/service/journals/00213/bibs/6128001/61280001.htm | archive-url = https://web.archive.org/web/20020112111228/http://link.springer.de/link/service/journals/00213/bibs/6128001/61280001.htm | url-status = dead | archive-date = 12 January 2002 }}</ref>唑吡坦对含有{{External_demand|wikipedia|GABRG1|γ-氨基丁酸受体亚基γ1|γ1}}和{{External_demand|wikipedia|GABRG3|γ-氨基丁酸受体亚基γ3|γ3}}亚基的受体没有亲和力,并且像绝大多数苯二氮卓类药物一样,它也对含有{{External_demand|wikipedia|Gamma-aminobutyric acid receptor subunit alpha-4|γ-氨基丁酸受体亚基α4|α4}}和{{External_demand|wikipedia|Gamma-aminobutyric acid receptor subunit alpha-6|γ-氨基丁酸受体亚基α6|α6}}亚基的受体缺乏亲和力。<ref name="pmid8794909">{{cite journal | vauthors = Wafford KA, Thompson SA, Thomas D, Sikela J, Wilcox AS, Whiting PJ | title = Functional characterization of human gamma-aminobutyric acidA receptors containing the alpha 4 subunit | journal = Molecular Pharmacology | volume = 50 | issue = 3 | pages = 670–8 | date = September 1996 | pmid = 8794909 | url = http://molpharm.aspetjournals.org/cgi/content/abstract/50/3/670 | access-date = 7 October 2007 | archive-date = 8 January 2009 | archive-url = https://web.archive.org/web/20090108105647/http://molpharm.aspetjournals.org/cgi/content/abstract/50/3/670 | url-status = dead }}</ref>唑吡坦或许是通过诱导受体构象来调节受体,它诱导的构象能够通过[[别构调节|正调节位点]]调节激动剂[[γ-氨基丁酸]]与其同源受体的结合强度,而不影响{{External_demand|wikipedia|Downregulation and upregulation|脱敏}}或峰值电流。<ref name="pmid9880578">{{cite journal | vauthors = Perrais D, Ropert N | title = Effect of zolpidem on miniature IPSCs and occupancy of postsynaptic GABAA receptors in central synapses | journal = The Journal of Neuroscience | volume = 19 | issue = 2 | pages = 578–88 | date = January 1999 | pmid = 9880578 | pmc = 6782193 | doi = 10.1523/JNEUROSCI.19-02-00578.1999}}</ref> |
||
与[[扎来普隆]]类似, 唑吡坦可能增加[[慢波睡眠]],但对第二睡眠期没有影响。<ref name="pmid15037809">{{cite journal | vauthors = Noguchi H, Kitazumi K, Mori M, Shiba T | title = Electroencephalographic properties of zaleplon, a non-benzodiazepine sedative/hypnotic, in rats | journal = Journal of Pharmacological Sciences | volume = 94 | issue = 3 | pages = 246–51 | date = March 2004 | pmid = 15037809 | doi = 10.1254/jphs.94.246 | quote = WARNING: The reference indicates that zaleplon-Sonata, not zolpidem, increases Slow-wave sleep | doi-access = free }}</ref>一项比较[[苯二氮卓类]]药物与[[非苯二氮卓类]]药物的文献综述显示,唑吡坦和苯二氮卓类药物在入睡潜伏期、总睡眠持续时间、觉醒次数、睡眠质量、不良事件、耐受性、失眠反弹和日间警觉性方面几乎没有显著的差异。<ref name="pmid15252823">{{cite journal | vauthors = Dündar Y, Dodd S, Strobl J, Boland A, Dickson R, Walley T | title = Comparative efficacy of newer hypnotic drugs for the short-term management of insomnia: a systematic review and meta-analysis | journal = Human Psychopharmacology | volume = 19 | issue = 5 | pages = 305–22 | date = July 2004 | pmid = 15252823 | doi = 10.1002/hup.594 | s2cid = 10888200 }}</ref> |
与[[扎来普隆]]类似, 唑吡坦可能增加[[慢波睡眠]],但对第二睡眠期没有影响。<ref name="pmid15037809">{{cite journal | vauthors = Noguchi H, Kitazumi K, Mori M, Shiba T | title = Electroencephalographic properties of zaleplon, a non-benzodiazepine sedative/hypnotic, in rats | journal = Journal of Pharmacological Sciences | volume = 94 | issue = 3 | pages = 246–51 | date = March 2004 | pmid = 15037809 | doi = 10.1254/jphs.94.246 | quote = WARNING: The reference indicates that zaleplon-Sonata, not zolpidem, increases Slow-wave sleep | doi-access = free }}</ref>一项比较[[苯二氮卓类]]药物与[[非苯二氮卓类]]药物的文献综述显示,唑吡坦和苯二氮卓类药物在入睡潜伏期、总睡眠持续时间、觉醒次数、睡眠质量、不良事件、耐受性、失眠反弹和日间警觉性方面几乎没有显著的差异。<ref name="pmid15252823">{{cite journal | vauthors = Dündar Y, Dodd S, Strobl J, Boland A, Dickson R, Walley T | title = Comparative efficacy of newer hypnotic drugs for the short-term management of insomnia: a systematic review and meta-analysis | journal = Human Psychopharmacology | volume = 19 | issue = 5 | pages = 305–22 | date = July 2004 | pmid = 15252823 | doi = 10.1002/hup.594 | s2cid = 10888200 }}</ref> |
||
| 第37行: | 第37行: | ||
微粒体研究表明唑吡坦由[[CYP3A4]](61%)、[[CYP2C9]](22%)、[[CYP1A2]](14%)、[[CYP2D6]](<3%)和[[CYP2C19]](<3%)代谢。<ref name="micrsome_liver">{{cite journal | vauthors = Von Moltke LL, Greenblatt DJ, Granda BW, Duan SX, Grassi JM, Venkatakrishnan K, Harmatz JS, Shader RI | display-authors = 6 | title = Zolpidem metabolism in vitro: responsible cytochromes, chemical inhibitors, and in vivo correlations | journal = British Journal of Clinical Pharmacology | volume = 48 | issue = 1 | pages = 89–97 | date = July 1999 | pmid = 10383565 | doi = 10.1046/j.1365-2125.1999.00953.x | pmc = 2014868 }}</ref>只有不到1%以原药形式从尿液排出。<ref name="costahalflife"/>唑吡坦的绝对生物利用度约为70%,在2小时内达到峰值浓度,在健康成年人中半衰期为2至3小时,<ref name=AHFS2018>{{cite web|title=Zolpidem (Monograph)|url=https://www.drugs.com/monograph/zolpidem-tartrate.html|publisher=The American Society of Health-System Pharmacists|date=27 April 2023|access-date=10 March 2024}}</ref><ref name="costahalflife"/>但在儿童中会缩短,在老年人和肝功能有问题的人群中延长。 |
微粒体研究表明唑吡坦由[[CYP3A4]](61%)、[[CYP2C9]](22%)、[[CYP1A2]](14%)、[[CYP2D6]](<3%)和[[CYP2C19]](<3%)代谢。<ref name="micrsome_liver">{{cite journal | vauthors = Von Moltke LL, Greenblatt DJ, Granda BW, Duan SX, Grassi JM, Venkatakrishnan K, Harmatz JS, Shader RI | display-authors = 6 | title = Zolpidem metabolism in vitro: responsible cytochromes, chemical inhibitors, and in vivo correlations | journal = British Journal of Clinical Pharmacology | volume = 48 | issue = 1 | pages = 89–97 | date = July 1999 | pmid = 10383565 | doi = 10.1046/j.1365-2125.1999.00953.x | pmc = 2014868 }}</ref>只有不到1%以原药形式从尿液排出。<ref name="costahalflife"/>唑吡坦的绝对生物利用度约为70%,在2小时内达到峰值浓度,在健康成年人中半衰期为2至3小时,<ref name=AHFS2018>{{cite web|title=Zolpidem (Monograph)|url=https://www.drugs.com/monograph/zolpidem-tartrate.html|publisher=The American Society of Health-System Pharmacists|date=27 April 2023|access-date=10 March 2024}}</ref><ref name="costahalflife"/>但在儿童中会缩短,在老年人和肝功能有问题的人群中延长。 |
||
== 法律地位 == |
|||
=== 中国大陆 === |
|||
唑吡坦在中国大陆于2013年被列入[[精神药品品种目]]第二类。 |
|||
==参考资料== |
==参考资料== |
||
| 第46行: | 第49行: | ||
== 外部引用 == |
== 外部引用 == |
||
{{External_demands}} |
{{External_demands}} |
||
{{中华人民共和国管制药品}} |
|||
{{DEFAULTSORT:Zolpidem}} |
{{DEFAULTSORT:Zolpidem}} |
||
2024年5月7日 (二) 15:14的最新版本
 | |
 | |
| 化学数据 | |
|---|---|
| 化学式 | C19H21N3O |
| 摩尔质量 | 307.40 g·mol−1 |
| 识别信息 | |
| IUPAC名称 | N,N,6-三甲基-2-(4-甲基苯基)-咪唑并(1,2-a)吡啶-3-乙酰胺 |
| CAS号 | |
| PubChem CID | |
唑吡坦(英语:Zolpidem)是一种Z-drugs安眠药,約在口服三十分钟之内起效,药效维持两至三小时。市场上常见的药名包括、Adormix、Ambien、Ambien CR、Edluar、Damixan、Ivedal、Nytamel、Stilnoct、Stilnox、Sucedal、Zoldem、Zolnod及Zolpihexal等,[1][2][3][4]中国大陆商品名为思诺思(Stilnox),台湾則有佐沛眠(Zolpium)、使蒂诺斯等名称。
药理学
唑吡坦是GABAA受体的正别构调节剂,它选择性结合GABAA受体的α1亚基,因此它具有较强的催眠和较弱的抗焦虑、肌肉松弛和抗惊厥特性。[5]与地西泮不同,唑吡坦可以与αβGABAA受体结合,它被证明与α1-α1界面结合。[6]唑吡坦对α2-和α3-亚基的亲和力比对α1低10倍,且对含α5亚基的受体没有表现出明显的亲和力。[7][8]含α1亚基的苯二氮卓位点(旧称ω1)主要存在于大脑中,而含α2亚基的苯二氮卓位点(旧称ω2)主要存在于脊柱中,因此唑吡坦更易于与位于大脑而不是脊柱中的GABAA受体结合。[9]唑吡坦对含有γ1和γ3亚基的受体没有亲和力,并且像绝大多数苯二氮卓类药物一样,它也对含有α4和α6亚基的受体缺乏亲和力。[10]唑吡坦或许是通过诱导受体构象来调节受体,它诱导的构象能够通过正调节位点调节激动剂γ-氨基丁酸与其同源受体的结合强度,而不影响脱敏或峰值电流。[11]
与扎来普隆类似, 唑吡坦可能增加慢波睡眠,但对第二睡眠期没有影响。[12]一项比较苯二氮卓类药物与非苯二氮卓类药物的文献综述显示,唑吡坦和苯二氮卓类药物在入睡潜伏期、总睡眠持续时间、觉醒次数、睡眠质量、不良事件、耐受性、失眠反弹和日间警觉性方面几乎没有显著的差异。[13]
药代动力学
微粒体研究表明唑吡坦由CYP3A4(61%)、CYP2C9(22%)、CYP1A2(14%)、CYP2D6(<3%)和CYP2C19(<3%)代谢。[14]只有不到1%以原药形式从尿液排出。[5]唑吡坦的绝对生物利用度约为70%,在2小时内达到峰值浓度,在健康成年人中半衰期为2至3小时,[15][5]但在儿童中会缩短,在老年人和肝功能有问题的人群中延长。
法律地位
中国大陆
唑吡坦在中国大陆于2013年被列入精神药品品种目第二类。
参考资料
- ↑ Ambien.com (2004). "AMBIEN Prescribing Information". Information About a Short-term Treatment for Insomnia - Ambien.com Home Page for Health-care Professionals. Sanofi-Synthelabo Inc. New York, NY 10016. Archived from the original on 2005-06-27. Retrieved 2005-06-27.
{{cite web}}: Unknown parameter|dead-url=ignored (|url-status=suggested) (help) - ↑ STILNOX (zolpidem tartrate) PRODUCT INFORMATION (页面存档备份,存于互联网档案馆) Sanofi-Synthelabo Australia Pty Limited. April 15, 2004
- ↑ "sanofi-aventis : Drugs and Products - CNS - Stilnox/Ambien/Myslee". 2006-11-07. Archived from the original on 2007-09-27. Retrieved 2006-11-22.
{{cite web}}: Unknown parameter|dead-url=ignored (|url-status=suggested) (help) - ↑ "Benzodiazepine Names". Archived from the original on 2008-12-08.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ↑ 5.0 5.1 5.2 Salvà P, Costa J (September 1995). "Clinical pharmacokinetics and pharmacodynamics of zolpidem. Therapeutic implications". Clinical Pharmacokinetics. 29 (3): 142–153. doi:10.2165/00003088-199529030-00002. PMID 8521677. S2CID 23391285.
- ↑ Che Has AT, Absalom N, van Nieuwenhuijzen PS, Clarkson AN, Ahring PK, Chebib M (June 2016). "Zolpidem is a potent stoichiometry-selective modulator of α1β3 GABAA receptors: evidence of a novel benzodiazepine site in the α1-α1 interface". Scientific Reports. 6: 28674. Bibcode:2016NatSR...628674C. doi:10.1038/srep28674. PMC 4921915. PMID 27346730.
- ↑ Pritchett DB, Seeburg PH (May 1990). "Gamma-aminobutyric acidA receptor alpha 5-subunit creates novel type II benzodiazepine receptor pharmacology". Journal of Neurochemistry. 54 (5): 1802–4. doi:10.1111/j.1471-4159.1990.tb01237.x. PMID 2157817. S2CID 86674799.
- ↑ Smith AJ, Alder L, Silk J, Adkins C, Fletcher AE, Scales T, Kerby J, Marshall G, Wafford KA, McKernan RM, Atack JR (May 2001). "Effect of alpha subunit on allosteric modulation of ion channel function in stably expressed human recombinant gamma-aminobutyric acid(A) receptors determined using (36)Cl ion flux". Molecular Pharmacology. 59 (5): 1108–18. doi:10.1124/mol.59.5.1108. PMID 11306694. S2CID 86156878.
- ↑ Rowlett JK, Woolverton WL (November 1996). "Assessment of benzodiazepine receptor heterogeneity in vivo: apparent pA2 and pKB analyses from behavioral studies". Psychopharmacology. 128 (1): 1–16. doi:10.1007/s002130050103. PMID 8944400. S2CID 25654504. Archived from the original on 12 January 2002.
- ↑ Wafford KA, Thompson SA, Thomas D, Sikela J, Wilcox AS, Whiting PJ (September 1996). "Functional characterization of human gamma-aminobutyric acidA receptors containing the alpha 4 subunit". Molecular Pharmacology. 50 (3): 670–8. PMID 8794909. Archived from the original on 8 January 2009. Retrieved 7 October 2007.
- ↑ Perrais D, Ropert N (January 1999). "Effect of zolpidem on miniature IPSCs and occupancy of postsynaptic GABAA receptors in central synapses". The Journal of Neuroscience. 19 (2): 578–88. doi:10.1523/JNEUROSCI.19-02-00578.1999. PMC 6782193. PMID 9880578.
- ↑ Noguchi H, Kitazumi K, Mori M, Shiba T (March 2004). "Electroencephalographic properties of zaleplon, a non-benzodiazepine sedative/hypnotic, in rats". Journal of Pharmacological Sciences. 94 (3): 246–51. doi:10.1254/jphs.94.246. PMID 15037809.
WARNING: The reference indicates that zaleplon-Sonata, not zolpidem, increases Slow-wave sleep
- ↑ Dündar Y, Dodd S, Strobl J, Boland A, Dickson R, Walley T (July 2004). "Comparative efficacy of newer hypnotic drugs for the short-term management of insomnia: a systematic review and meta-analysis". Human Psychopharmacology. 19 (5): 305–22. doi:10.1002/hup.594. PMID 15252823. S2CID 10888200.
- ↑ Von Moltke LL, Greenblatt DJ, Granda BW, Duan SX, Grassi JM, Venkatakrishnan K, et al. (July 1999). "Zolpidem metabolism in vitro: responsible cytochromes, chemical inhibitors, and in vivo correlations". British Journal of Clinical Pharmacology. 48 (1): 89–97. doi:10.1046/j.1365-2125.1999.00953.x. PMC 2014868. PMID 10383565.
- ↑ "Zolpidem (Monograph)". The American Society of Health-System Pharmacists. 27 April 2023. Retrieved 10 March 2024.
外部链接
- "Zolpidem". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2020-11-27. Retrieved 2020-10-22.
{{cite web}}: Unknown parameter|dead-url=ignored (|url-status=suggested) (help)
外部引用
此条目使用了来自外部的条目,它们可能需要在站内被创建或编辑