N,N-二甲基色胺:修订间差异
小 修正错字 |
小无编辑摘要 |
||
| (未显示同一用户的3个中间版本) | |||
| 第1行: | 第1行: | ||
{{Title|<i>N</i>,<i>N</i>-二甲基色胺}} |
{{Title|<i>N</i>,<i>N</i>-二甲基色胺}} |
||
{{Drugbox |
{{Drugbox |
||
| image = |
| image = Structure_of_N,N-Dimethyltryptamine.png |
||
| image2 = |
| image2 = N,N-Dimethyltryptamine_molecule_spacefill.png |
||
<!-- 化学数据 --> |
<!-- 化学数据 --> |
||
| 第10行: | 第10行: | ||
| IUPAC_name = 2-(1H-吲哚-3-基)-N,N-二甲基乙胺 |
| IUPAC_name = 2-(1H-吲哚-3-基)-N,N-二甲基乙胺 |
||
| CAS_number = 61-50-7 |
| CAS_number = 61-50-7 |
||
| PubChem = 6089 |
|||
<!-- 药理数据 --> |
|||
| Pharmacology_refs = <ref name="pmid27216487">{{cite journal | vauthors = Rickli A, Moning OD, Hoener MC, Liechti ME | title = Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens | journal = European Neuropsychopharmacology | volume = 26 | issue = 8 | pages = 1327–1337 | date = August 2016 | pmid = 27216487 | doi = 10.1016/j.euroneuro.2016.05.001 | s2cid = 6685927 | url = http://edoc.unibas.ch/53326/1/20170117174852_587e4af45b658.pdf }}</ref> |
|||
| 5HT1A_action = agonist |
|||
| 5HT1A_Ki = 0.075 |
|||
| 5HT1B_Ki = ? |
|||
| 5HT1D_Ki = ? |
|||
| 5HT2A_action = agonist |
|||
| 5HT2A_Function_selective = PLA2 |
|||
| 5HT2A_Ki = 0.237 |
|||
| 5HT2B_Ki = ? |
|||
| 5HT2C_action = agonist |
|||
| 5HT2C_Ki = 0.424 |
|||
| DRD1_Ki = 6 |
|||
| DRD2_Ki = 3 |
|||
| DRD3_Ki = 6.3 |
|||
| HRH1_Ki = 0.22 |
|||
| SERT_action = blocker |
|||
| SERT_Ki = 6 |
|||
| DAT_Ki = 22 |
|||
| NET_Ki = 6.5 |
|||
| ADRA1A_Ki = 1.3 |
|||
| ADRA2A_Ki = 2.1 |
|||
| TAAR1_Ki = 2.2 |
|||
| I1_Ki = ? |
|||
}} |
}} |
||
| 第17行: | 第43行: | ||
DMT以低于0.6μmol/L的亲和力非选择性地与以下血清素受体结合:[[血清素1A受体|5-HT<sub>1A</sub>]]、<ref name="pmid19881490">{{cite journal | vauthors = Keiser MJ, Setola V, Irwin JJ, Laggner C, Abbas AI, Hufeisen SJ, Jensen NH, Kuijer MB, Matos RC, Tran TB, Whaley R, Glennon RA, Hert J, Thomas KL, Edwards DD, Shoichet BK, Roth BL | title = Predicting new molecular targets for known drugs | journal = Nature | volume = 462 | issue = 7270 | pages = 175–181 | date = November 2009 | pmid = 19881490 | pmc = 2784146 | doi = 10.1038/nature08506 | bibcode = 2009Natur.462..175K }}</ref><ref name="pmid1828347">{{cite journal | vauthors = Deliganis AV, Pierce PA, Peroutka SJ | title = Differential interactions of dimethyltryptamine (DMT) with 5-HT<sub>1A</sub> and 5-HT<sub>2</sub> receptors | journal = Biochemical Pharmacology | volume = 41 | issue = 11 | pages = 1739–1744 | date = June 1991 | pmid = 1828347 | doi = 10.1016/0006-2952(91)90178-8 }}</ref><ref name="pmid2540505">{{cite journal | vauthors = Pierce PA, Peroutka SJ | title = Hallucinogenic drug interactions with neurotransmitter receptor binding sites in human cortex | journal = Psychopharmacology | volume = 97 | issue = 1 | pages = 118–122 | year = 1989 | pmid = 2540505 | doi = 10.1007/BF00443425 | s2cid = 32936434 }}</ref> [[血清素1B受体|5-HT<sub>1B</sub>]]、<ref name="pmid19881490" /><ref name="pmid20126400">{{cite journal | vauthors = Ray TS | title = Psychedelics and the human receptorome | journal = PLOS ONE | volume = 5 | issue = 2 | pages = e9019 | date = February 2010 | pmid = 20126400 | pmc = 2814854 | doi = 10.1371/journal.pone.0009019 | bibcode = 2010PLoSO...5.9019R | doi-access = free }}</ref> [[血清素1D受体|5-HT<sub>1D</sub>]]、<ref name="pmid19881490" /><ref name="pmid2540505" /><ref name="pmid20126400" /> [[血清素2A受体|5-HT<sub>2A</sub>]]、 |
DMT以低于0.6μmol/L的亲和力非选择性地与以下血清素受体结合:[[血清素1A受体|5-HT<sub>1A</sub>]]、<ref name="pmid19881490">{{cite journal | vauthors = Keiser MJ, Setola V, Irwin JJ, Laggner C, Abbas AI, Hufeisen SJ, Jensen NH, Kuijer MB, Matos RC, Tran TB, Whaley R, Glennon RA, Hert J, Thomas KL, Edwards DD, Shoichet BK, Roth BL | title = Predicting new molecular targets for known drugs | journal = Nature | volume = 462 | issue = 7270 | pages = 175–181 | date = November 2009 | pmid = 19881490 | pmc = 2784146 | doi = 10.1038/nature08506 | bibcode = 2009Natur.462..175K }}</ref><ref name="pmid1828347">{{cite journal | vauthors = Deliganis AV, Pierce PA, Peroutka SJ | title = Differential interactions of dimethyltryptamine (DMT) with 5-HT<sub>1A</sub> and 5-HT<sub>2</sub> receptors | journal = Biochemical Pharmacology | volume = 41 | issue = 11 | pages = 1739–1744 | date = June 1991 | pmid = 1828347 | doi = 10.1016/0006-2952(91)90178-8 }}</ref><ref name="pmid2540505">{{cite journal | vauthors = Pierce PA, Peroutka SJ | title = Hallucinogenic drug interactions with neurotransmitter receptor binding sites in human cortex | journal = Psychopharmacology | volume = 97 | issue = 1 | pages = 118–122 | year = 1989 | pmid = 2540505 | doi = 10.1007/BF00443425 | s2cid = 32936434 }}</ref> [[血清素1B受体|5-HT<sub>1B</sub>]]、<ref name="pmid19881490" /><ref name="pmid20126400">{{cite journal | vauthors = Ray TS | title = Psychedelics and the human receptorome | journal = PLOS ONE | volume = 5 | issue = 2 | pages = e9019 | date = February 2010 | pmid = 20126400 | pmc = 2814854 | doi = 10.1371/journal.pone.0009019 | bibcode = 2010PLoSO...5.9019R | doi-access = free }}</ref> [[血清素1D受体|5-HT<sub>1D</sub>]]、<ref name="pmid19881490" /><ref name="pmid2540505" /><ref name="pmid20126400" /> [[血清素2A受体|5-HT<sub>2A</sub>]]、 |
||
<ref name="pmid19881490" /><ref name="pmid2540505" /><ref name="pmid20126400" /><ref name="pmid9768567">{{cite journal | vauthors = Smith RL, Canton H, Barrett RJ, Sanders-Bush E | title = Agonist properties of ''N'',''N''-dimethyltryptamine at serotonin 5-HT<sub>2A</sub> and 5-HT<sub>2C</sub> receptors | journal = Pharmacology, Biochemistry, and Behavior | volume = 61 | issue = 3 | pages = 323–330 | date = November 1998 | pmid = 9768567 | doi = 10.1016/S0091-3057(98)00110-5 | s2cid = 27591297 | url = http://crfdl.org:1111/xmlui/bitstream/handle/123456789/17/Agonist%20Properties%20of%20N,N-Dimethyltryptaminenext%20term%20at%20Ser.pdf }}{{Dead link|date=July 2018 |bot=InternetArchiveBot |fix-attempted=yes }}</ref> [[血清素2B受体|5-HT<sub>2B</sub>]]、<ref name="pmid19881490" /><ref name="pmid20126400" /> [[血清素2C受体|5-HT<sub>2C</sub>]]、<ref name="pmid19881490" /><ref name="pmid20126400" /><ref name="pmid9768567" /> [[血清素6受体|5-HT<sub>6</sub>]]、<ref name="pmid19881490" /><ref name="pmid20126400" />[[血清素7受体|5-HT<sub>7</sub>]]。<ref name="pmid19881490" /><ref name="pmid20126400" />它在5-HT1A、<ref name="pmid1828347" />5-HT2A和5-HT2C上作为激动剂起效。<ref name="pmid19881490" /><ref name="pmid20126400" /><ref name="pmid9768567" />它对其他血清素受体的[[内在活性|活性]]仍有待确定。特别令人感兴趣的是确定其对人类5-HT<sub>2B</sub>受体的功效,因为两项体外测定证明了DMT对该受体具有0.108 µmol/L、<ref name="pmid20126400" />0.184 µmol/L<ref name="pmid19881490" />的高亲和力。长期或频繁使用对5-HT<sub>2B</sub>受体表现出优先高亲和力和明显激动作用的血清素药物与[[瓣膜性心脏病]]存在因果关系。<ref name="pmid19505264">{{cite journal | vauthors = Rothman RB, Baumann MH | title = Serotonergic drugs and valvular heart disease | journal = Expert Opinion on Drug Safety | volume = 8 | issue = 3 | pages = 317–329 | date = May 2009 | pmid = 19505264 | pmc = 2695569 | doi = 10.1517/14740330902931524 }}</ref><ref name="pmid17202450">{{cite journal|author1-link=Bryan Roth | vauthors = Roth BL | title = Drugs and valvular heart disease | journal = The New England Journal of Medicine | volume = 356 | issue = 1 | pages = 6–9 | date = January 2007 | pmid = 17202450 | doi = 10.1056/NEJMp068265 }}</ref><ref>{{cite journal | vauthors = Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB | title = Functional selectivity and classical concepts of quantitative pharmacology | journal = The Journal of Pharmacology and Experimental Therapeutics | volume = 320 | issue = 1 | pages = 1–13 | date = January 2007 | pmid = 16803859 | doi = 10.1124/jpet.106.104463 | s2cid = 447937 | url = https://cdr.lib.unc.edu/concern/articles/xs55mf307 }}</ref>它还被证明对[[多巴胺]][[多巴胺D1受体|D<sub>1</sub>]]、[[肾上腺素α1受体|肾上腺素能α<sub>1</sub>]]、[[肾上腺素α2受体|肾上腺素α<sub>2</sub>]]、{{External_demand|wikipedia|Imidazoline receptor|咪唑啉受体|咪唑啉-1}}和[[σ-1受体|σ<sub>1</sub>]][[受体(生物化学)|受体]]具有亲和力。<ref name="pmid2540505" /><ref name="pmid20126400" /><ref name="pmid16962229">{{cite journal | vauthors = Burchett SA, Hicks TP | title = The mysterious trace amines: protean neuromodulators of synaptic transmission in mammalian brain | journal = Progress in Neurobiology | volume = 79 | issue = 5–6 | pages = 223–246 | date = August 2006 | pmid = 16962229 | doi = 10.1016/j.pneurobio.2006.07.003 | s2cid = 10272684 | url = http://www.mimosahostilis.com/files/The%20mysterious%20trace%20amines%20%20protean%20neuromodulato.pdf | url-status = dead | df = dmy-all | oclc = 231983957 | archive-date = 1 February 2012 | archive-url = https://web.archive.org/web/20120201112618/http://www.mimosahostilis.com/files/The%20mysterious%20trace%20amines%20%20protean%20neuromodulato.pdf }}</ref> 多个证据证明σ<sub>1</sub>受体在浓度为50~100 μmol/L时被激活,<ref name="pmid19213917">{{cite journal | vauthors = Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE | title = The hallucinogen ''N'',''N''-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator | journal = Science | volume = 323 | issue = 5916 | pages = 934–937 | date = February 2009 | pmid = 19213917 | pmc = 2947205 | doi = 10.1126/science.1166127 | bibcode = 2009Sci...323..934F }}</ref>而在其他受体结合位点的效力尚不清楚。它还在体外被证明是人类血小板中表达的细胞表面{{External demand|wikipedia|serotonin transporter|血清素转运蛋白}}(SERT)的[[底物(生物化学)|底物]]、大鼠{{External demand|wikipedia|vesicular monoamine transporter 2|囊泡单胺转运蛋白2}}(VMAT2)、 [[秋粘虫]]的Sf9细胞中瞬时表达。DMT在血小板抑制SERT回收血清素的平均浓度为4±0.70 µmol/L,抑制VMAT2血清素回收的平均浓度为93±6.8 µmol/L。<ref name="pmid19756361">{{cite journal | vauthors = Cozzi NV, Gopalakrishnan A, Anderson LL, Feih JT, Shulgin AT, Daley PF, Ruoho AE | title = Dimethyltryptamine and other hallucinogenic tryptamines exhibit substrate behavior at the serotonin uptake transporter and the vesicle monoamine transporter | journal = Journal of Neural Transmission | volume = 116 | issue = 12 | pages = 1591–1599 | date = December 2009 | pmid = 19756361 | doi = 10.1007/s00702-009-0308-8 | s2cid = 15928043 | url = http://www.neurophys.wisc.edu/~cozzi/Hallucinogenic%20tryptamines%20as%20SERT%20and%20VMAT2%20substrates.%20%20Cozzi.%20%20J.%20Neural%20Transm.,%20116,%201591-1599%20(2009).pdf | access-date = 20 November 2010 | url-status = dead | archive-url = https://web.archive.org/web/20100617172010/http://www.neurophys.wisc.edu/~cozzi/Hallucinogenic%20tryptamines%20as%20SERT%20and%20VMAT2%20substrates.%20%20Cozzi.%20%20J.%20Neural%20Transm.,%20116,%201591-1599%20(2009).pdf | archive-date = 17 June 2010 }}</ref>与其他所谓的“经典致幻剂”一样,<ref name="nida1994">{{cite book | vauthors = Glennon RA | veditors = Lin GC, Glennon RA |title=Hallucinogens: An Update |chapter-url=http://crfdl.org:1111/xmlui/bitstream/handle/123456789/288/hallucinogens%20an%20update.pdf |archive-url=https://web.archive.org/web/20110725203539/http://crfdl.org:1111/xmlui/bitstream/handle/123456789/288/hallucinogens%20an%20update.pdf |archive-date=2011-07-25 |url-status=live |series=NIDA Research Monograph Series |volume=146 |year=1994 |publisher=U.S. Dept. of Health and Human Services, Public Health Service, National Institutes of Health, National Institute on Drug Abuse |location=Rockville, MD |page=4 |chapter=Classical hallucinogens: an introductory overview}}{{Dead link|date=July 2018 |bot=InternetArchiveBot |fix-attempted=yes }}</ref>DMT的致幻作用的很大一部分可归因于5-HT<sub>2A</sub>受体的[[功能选择性]]激活。<ref name="pmid8297216">{{cite journal | vauthors = Strassman RJ, Qualls CR | title = Dose-response study of ''N'',''N''-dimethyltryptamine in humans. I. Neuroendocrine, autonomic, and cardiovascular effects | journal = Archives of General Psychiatry | volume = 51 | issue = 2 | pages = 85–97 | date = February 1994 | pmid = 8297216 | doi = 10.1001/archpsyc.1994.03950020009001 }}</ref><ref name="pmid19881490" /><ref name="pmid17977517">{{cite journal | vauthors = Fantegrossi WE, Murnane KS, Reissig CJ | title = The behavioral pharmacology of hallucinogens | journal = Biochemical Pharmacology | volume = 75 | issue = 1 | pages = 17–33 | date = January 2008 | pmid = 17977517 | pmc = 2247373 | doi = 10.1016/j.bcp.2007.07.018 }}</ref><ref name="pmid14761703">{{cite journal | vauthors = Nichols DE | title = Hallucinogens | journal = Pharmacology & Therapeutics | volume = 101 | issue = 2 | pages = 131–181 | date = February 2004 | pmid = 14761703 | doi = 10.1016/j.pharmthera.2003.11.002 }}</ref><ref name="pmid9875725">{{cite journal | vauthors = Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, Vogel H, Hell D | title = Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action | journal = NeuroReport | volume = 9 | issue = 17 | pages = 3897–3902 | date = December 1998 | pmid = 9875725 | doi = 10.1097/00001756-199812010-00024 | s2cid = 37706068 }}</ref><ref name="pmid8788488">{{cite journal | vauthors = Strassman RJ | title = Human psychopharmacology of ''N'',''N''-dimethyltryptamine | journal = Behavioural Brain Research | volume = 73 | issue = 1–2 | pages = 121–124 | year = 1996 | pmid = 8788488 | doi = 10.1016/0166-4328(96)00081-2 | s2cid = 4047951 | url = http://crfdl.org:1111/xmlui/bitstream/handle/123456789/373/Beh_Brain_Res_96.pdf }}{{Dead link|date=July 2018 |bot=InternetArchiveBot |fix-attempted=yes }}</ref><ref name="pmid6513725">{{cite journal | vauthors = Glennon RA, Titeler M, McKenney JD | title = Evidence for 5-HT<sub>2</sub> involvement in the mechanism of action of hallucinogenic agents | journal = Life Sciences | volume = 35 | issue = 25 | pages = 2505–2511 | date = December 1984 | pmid = 6513725 | doi = 10.1016/0024-3205(84)90436-3 }}</ref>DMT浓度在体外对人类5-HT<sub>2A</sub>受体产生50%的最大效应([[EC50|EC<sub>50</sub>]])的范围为0.118~0.983 µmol/L,<ref name="pmid19881490" /><ref name="pmid20126400" /><ref name="pmid9768567" /><ref name="pmid9023266">{{cite journal | vauthors = Roth BL, Choudhary MS, Khan N, Uluer AZ | title = High-affinity agonist binding is not sufficient for agonist efficacy at 5-hydroxytryptamine2A receptors: evidence in favor of a modified ternary complex model | journal = The Journal of Pharmacology and Experimental Therapeutics | volume = 280 | issue = 2 | pages = 576–83 | date = February 1997 | pmid = 9023266 | url = http://jpet.aspetjournals.org/content/280/2/576.full.pdf }}</ref> 该值范围与给予完全迷幻剂量后在血液和血浆中测量的浓度范围非常吻合。由于DMT已被证明对人类血清素2C受体的功效 (EC<sub>50</sub>) 略高于对2A受体的功效,<ref name="pmid20126400" /><ref name="pmid9768567" /> 因此5-HT<sub>2C</sub>也可能对DMT的整体效果有影响。<ref name="pmid14761703" /><ref name="pmid20165943">{{cite journal | vauthors = Canal CE, Olaghere da Silva UB, Gresch PJ, Watt EE, Sanders-Bush E, Airey DC | title = The serotonin 2C receptor potently modulates the head-twitch response in mice induced by a phenethylamine hallucinogen | journal = Psychopharmacology | volume = 209 | issue = 2 | pages = 163–174 | date = April 2010 | pmid = 20165943 | pmc = 2868321 | doi = 10.1007/s00213-010-1784-0 }}</ref>其他受体例如5-HT<sub>1A</sub><ref name="pmid2540505" /><ref name="pmid14761703" /><ref name="pmid8788488" />、σ<sub>1</sub><ref name="pmid19213917" /><ref name="pmid19278957">{{cite journal | vauthors = Su TP, Hayashi T, Vaupel DB | title = When the endogenous hallucinogenic trace amine ''N'',''N''-dimethyltryptamine meets the sigma-1 receptor | journal = Science Signaling | volume = 2 | issue = 61 | pages = pe12 | date = March 2009 | pmid = 19278957 | pmc = 3155724 | doi = 10.1126/scisignal.261pe12 }}</ref>等也可能发挥作用。 |
<ref name="pmid19881490" /><ref name="pmid2540505" /><ref name="pmid20126400" /><ref name="pmid9768567">{{cite journal | vauthors = Smith RL, Canton H, Barrett RJ, Sanders-Bush E | title = Agonist properties of ''N'',''N''-dimethyltryptamine at serotonin 5-HT<sub>2A</sub> and 5-HT<sub>2C</sub> receptors | journal = Pharmacology, Biochemistry, and Behavior | volume = 61 | issue = 3 | pages = 323–330 | date = November 1998 | pmid = 9768567 | doi = 10.1016/S0091-3057(98)00110-5 | s2cid = 27591297 | url = http://crfdl.org:1111/xmlui/bitstream/handle/123456789/17/Agonist%20Properties%20of%20N,N-Dimethyltryptaminenext%20term%20at%20Ser.pdf }}{{Dead link|date=July 2018 |bot=InternetArchiveBot |fix-attempted=yes }}</ref> [[血清素2B受体|5-HT<sub>2B</sub>]]、<ref name="pmid19881490" /><ref name="pmid20126400" /> [[血清素2C受体|5-HT<sub>2C</sub>]]、<ref name="pmid19881490" /><ref name="pmid20126400" /><ref name="pmid9768567" /> [[血清素6受体|5-HT<sub>6</sub>]]、<ref name="pmid19881490" /><ref name="pmid20126400" />[[血清素7受体|5-HT<sub>7</sub>]]。<ref name="pmid19881490" /><ref name="pmid20126400" />它在5-HT1A、<ref name="pmid1828347" />5-HT2A和5-HT2C上作为激动剂起效。<ref name="pmid19881490" /><ref name="pmid20126400" /><ref name="pmid9768567" />它对其他血清素受体的[[内在活性|活性]]仍有待确定。特别令人感兴趣的是确定其对人类5-HT<sub>2B</sub>受体的功效,因为两项体外测定证明了DMT对该受体具有0.108 µmol/L、<ref name="pmid20126400" />0.184 µmol/L<ref name="pmid19881490" />的高亲和力。长期或频繁使用对5-HT<sub>2B</sub>受体表现出优先高亲和力和明显激动作用的血清素药物与[[瓣膜性心脏病]]存在因果关系。<ref name="pmid19505264">{{cite journal | vauthors = Rothman RB, Baumann MH | title = Serotonergic drugs and valvular heart disease | journal = Expert Opinion on Drug Safety | volume = 8 | issue = 3 | pages = 317–329 | date = May 2009 | pmid = 19505264 | pmc = 2695569 | doi = 10.1517/14740330902931524 }}</ref><ref name="pmid17202450">{{cite journal|author1-link=Bryan Roth | vauthors = Roth BL | title = Drugs and valvular heart disease | journal = The New England Journal of Medicine | volume = 356 | issue = 1 | pages = 6–9 | date = January 2007 | pmid = 17202450 | doi = 10.1056/NEJMp068265 }}</ref><ref>{{cite journal | vauthors = Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB | title = Functional selectivity and classical concepts of quantitative pharmacology | journal = The Journal of Pharmacology and Experimental Therapeutics | volume = 320 | issue = 1 | pages = 1–13 | date = January 2007 | pmid = 16803859 | doi = 10.1124/jpet.106.104463 | s2cid = 447937 | url = https://cdr.lib.unc.edu/concern/articles/xs55mf307 }}</ref>它还被证明对[[多巴胺]][[多巴胺D1受体|D<sub>1</sub>]]、[[肾上腺素α1受体|肾上腺素能α<sub>1</sub>]]、[[肾上腺素α2受体|肾上腺素α<sub>2</sub>]]、{{External_demand|wikipedia|Imidazoline receptor|咪唑啉受体|咪唑啉-1}}和[[σ-1受体|σ<sub>1</sub>]][[受体(生物化学)|受体]]具有亲和力。<ref name="pmid2540505" /><ref name="pmid20126400" /><ref name="pmid16962229">{{cite journal | vauthors = Burchett SA, Hicks TP | title = The mysterious trace amines: protean neuromodulators of synaptic transmission in mammalian brain | journal = Progress in Neurobiology | volume = 79 | issue = 5–6 | pages = 223–246 | date = August 2006 | pmid = 16962229 | doi = 10.1016/j.pneurobio.2006.07.003 | s2cid = 10272684 | url = http://www.mimosahostilis.com/files/The%20mysterious%20trace%20amines%20%20protean%20neuromodulato.pdf | url-status = dead | df = dmy-all | oclc = 231983957 | archive-date = 1 February 2012 | archive-url = https://web.archive.org/web/20120201112618/http://www.mimosahostilis.com/files/The%20mysterious%20trace%20amines%20%20protean%20neuromodulato.pdf }}</ref> 多个证据证明σ<sub>1</sub>受体在浓度为50~100 μmol/L时被激活,<ref name="pmid19213917">{{cite journal | vauthors = Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE | title = The hallucinogen ''N'',''N''-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator | journal = Science | volume = 323 | issue = 5916 | pages = 934–937 | date = February 2009 | pmid = 19213917 | pmc = 2947205 | doi = 10.1126/science.1166127 | bibcode = 2009Sci...323..934F }}</ref>而在其他受体结合位点的效力尚不清楚。它还在体外被证明是人类血小板中表达的细胞表面{{External demand|wikipedia|serotonin transporter|血清素转运蛋白}}(SERT)的[[底物(生物化学)|底物]]、大鼠{{External demand|wikipedia|vesicular monoamine transporter 2|囊泡单胺转运蛋白2}}(VMAT2)、 [[秋粘虫]]的Sf9细胞中瞬时表达。DMT在血小板抑制SERT回收血清素的平均浓度为4±0.70 µmol/L,抑制VMAT2血清素回收的平均浓度为93±6.8 µmol/L。<ref name="pmid19756361">{{cite journal | vauthors = Cozzi NV, Gopalakrishnan A, Anderson LL, Feih JT, Shulgin AT, Daley PF, Ruoho AE | title = Dimethyltryptamine and other hallucinogenic tryptamines exhibit substrate behavior at the serotonin uptake transporter and the vesicle monoamine transporter | journal = Journal of Neural Transmission | volume = 116 | issue = 12 | pages = 1591–1599 | date = December 2009 | pmid = 19756361 | doi = 10.1007/s00702-009-0308-8 | s2cid = 15928043 | url = http://www.neurophys.wisc.edu/~cozzi/Hallucinogenic%20tryptamines%20as%20SERT%20and%20VMAT2%20substrates.%20%20Cozzi.%20%20J.%20Neural%20Transm.,%20116,%201591-1599%20(2009).pdf | access-date = 20 November 2010 | url-status = dead | archive-url = https://web.archive.org/web/20100617172010/http://www.neurophys.wisc.edu/~cozzi/Hallucinogenic%20tryptamines%20as%20SERT%20and%20VMAT2%20substrates.%20%20Cozzi.%20%20J.%20Neural%20Transm.,%20116,%201591-1599%20(2009).pdf | archive-date = 17 June 2010 }}</ref>与其他所谓的“经典致幻剂”一样,<ref name="nida1994">{{cite book | vauthors = Glennon RA | veditors = Lin GC, Glennon RA |title=Hallucinogens: An Update |chapter-url=http://crfdl.org:1111/xmlui/bitstream/handle/123456789/288/hallucinogens%20an%20update.pdf |archive-url=https://web.archive.org/web/20110725203539/http://crfdl.org:1111/xmlui/bitstream/handle/123456789/288/hallucinogens%20an%20update.pdf |archive-date=2011-07-25 |url-status=live |series=NIDA Research Monograph Series |volume=146 |year=1994 |publisher=U.S. Dept. of Health and Human Services, Public Health Service, National Institutes of Health, National Institute on Drug Abuse |location=Rockville, MD |page=4 |chapter=Classical hallucinogens: an introductory overview}}{{Dead link|date=July 2018 |bot=InternetArchiveBot |fix-attempted=yes }}</ref>DMT的致幻作用的很大一部分可归因于5-HT<sub>2A</sub>受体的[[功能选择性]]激活。<ref name="pmid8297216">{{cite journal | vauthors = Strassman RJ, Qualls CR | title = Dose-response study of ''N'',''N''-dimethyltryptamine in humans. I. Neuroendocrine, autonomic, and cardiovascular effects | journal = Archives of General Psychiatry | volume = 51 | issue = 2 | pages = 85–97 | date = February 1994 | pmid = 8297216 | doi = 10.1001/archpsyc.1994.03950020009001 }}</ref><ref name="pmid19881490" /><ref name="pmid17977517">{{cite journal | vauthors = Fantegrossi WE, Murnane KS, Reissig CJ | title = The behavioral pharmacology of hallucinogens | journal = Biochemical Pharmacology | volume = 75 | issue = 1 | pages = 17–33 | date = January 2008 | pmid = 17977517 | pmc = 2247373 | doi = 10.1016/j.bcp.2007.07.018 }}</ref><ref name="pmid14761703">{{cite journal | vauthors = Nichols DE | title = Hallucinogens | journal = Pharmacology & Therapeutics | volume = 101 | issue = 2 | pages = 131–181 | date = February 2004 | pmid = 14761703 | doi = 10.1016/j.pharmthera.2003.11.002 }}</ref><ref name="pmid9875725">{{cite journal | vauthors = Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, Vogel H, Hell D | title = Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action | journal = NeuroReport | volume = 9 | issue = 17 | pages = 3897–3902 | date = December 1998 | pmid = 9875725 | doi = 10.1097/00001756-199812010-00024 | s2cid = 37706068 }}</ref><ref name="pmid8788488">{{cite journal | vauthors = Strassman RJ | title = Human psychopharmacology of ''N'',''N''-dimethyltryptamine | journal = Behavioural Brain Research | volume = 73 | issue = 1–2 | pages = 121–124 | year = 1996 | pmid = 8788488 | doi = 10.1016/0166-4328(96)00081-2 | s2cid = 4047951 | url = http://crfdl.org:1111/xmlui/bitstream/handle/123456789/373/Beh_Brain_Res_96.pdf }}{{Dead link|date=July 2018 |bot=InternetArchiveBot |fix-attempted=yes }}</ref><ref name="pmid6513725">{{cite journal | vauthors = Glennon RA, Titeler M, McKenney JD | title = Evidence for 5-HT<sub>2</sub> involvement in the mechanism of action of hallucinogenic agents | journal = Life Sciences | volume = 35 | issue = 25 | pages = 2505–2511 | date = December 1984 | pmid = 6513725 | doi = 10.1016/0024-3205(84)90436-3 }}</ref>DMT浓度在体外对人类5-HT<sub>2A</sub>受体产生50%的最大效应([[EC50|EC<sub>50</sub>]])的范围为0.118~0.983 µmol/L,<ref name="pmid19881490" /><ref name="pmid20126400" /><ref name="pmid9768567" /><ref name="pmid9023266">{{cite journal | vauthors = Roth BL, Choudhary MS, Khan N, Uluer AZ | title = High-affinity agonist binding is not sufficient for agonist efficacy at 5-hydroxytryptamine2A receptors: evidence in favor of a modified ternary complex model | journal = The Journal of Pharmacology and Experimental Therapeutics | volume = 280 | issue = 2 | pages = 576–83 | date = February 1997 | pmid = 9023266 | url = http://jpet.aspetjournals.org/content/280/2/576.full.pdf }}</ref> 该值范围与给予完全迷幻剂量后在血液和血浆中测量的浓度范围非常吻合。由于DMT已被证明对人类血清素2C受体的功效 (EC<sub>50</sub>) 略高于对2A受体的功效,<ref name="pmid20126400" /><ref name="pmid9768567" /> 因此5-HT<sub>2C</sub>也可能对DMT的整体效果有影响。<ref name="pmid14761703" /><ref name="pmid20165943">{{cite journal | vauthors = Canal CE, Olaghere da Silva UB, Gresch PJ, Watt EE, Sanders-Bush E, Airey DC | title = The serotonin 2C receptor potently modulates the head-twitch response in mice induced by a phenethylamine hallucinogen | journal = Psychopharmacology | volume = 209 | issue = 2 | pages = 163–174 | date = April 2010 | pmid = 20165943 | pmc = 2868321 | doi = 10.1007/s00213-010-1784-0 }}</ref>其他受体例如5-HT<sub>1A</sub><ref name="pmid2540505" /><ref name="pmid14761703" /><ref name="pmid8788488" />、σ<sub>1</sub><ref name="pmid19213917" /><ref name="pmid19278957">{{cite journal | vauthors = Su TP, Hayashi T, Vaupel DB | title = When the endogenous hallucinogenic trace amine ''N'',''N''-dimethyltryptamine meets the sigma-1 receptor | journal = Science Signaling | volume = 2 | issue = 61 | pages = pe12 | date = March 2009 | pmid = 19278957 | pmc = 3155724 | doi = 10.1126/scisignal.261pe12 }}</ref>等也可能发挥作用。 |
||
{| class="wikitable" |
|||
|- |
|||
! 受体 |
|||
! ''亲和力 K<sub>i</sub>'' (μM)<ref name="pmid27216487">{{cite journal | vauthors = Rickli A, Moning OD, Hoener MC, Liechti ME | title = Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens | journal = European Neuropsychopharmacology | volume = 26 | issue = 8 | pages = 1327–1337 | date = August 2016 | pmid = 27216487 | doi = 10.1016/j.euroneuro.2016.05.001 | s2cid = 6685927 | url = http://edoc.unibas.ch/53326/1/20170117174852_587e4af45b658.pdf }}</ref> |
|||
!行为 |
|||
|- |
|||
| [[血清素1A受体|5-HT<sub>1A</sub>]] |
|||
| 0.075 |
|||
|↑ |
|||
|- |
|||
| [[血清素1B受体|5-HT<sub>1B</sub>]] |
|||
| ? |
|||
| |
|||
|- |
|||
| [[血清素1D受体|5-HT<sub>1D</sub>]] |
|||
| ? |
|||
| |
|||
|- |
|||
| [[血清素2A受体|5-HT<sub>2A</sub>]] |
|||
| 0.237 |
|||
|↑ |
|||
|- |
|||
| [[血清素2B受体|5-HT<sub>2B</sub>]] |
|||
| ? |
|||
| |
|||
|- |
|||
| [[血清素2C受体|5-HT<sub>2C</sub>]] |
|||
| 0.424 |
|||
|↑ |
|||
|- |
|||
| [[血清素6受体|5-HT<sub>6</sub>]] |
|||
| ? |
|||
| |
|||
|- |
|||
| [[血清素7受体|5-HT<sub>7</sub>]] |
|||
| ? |
|||
| |
|||
|- |
|||
| [[多巴胺D1受体|D<sub>1</sub>]] |
|||
| 6 |
|||
| |
|||
|- |
|||
| [[多巴胺D2受体|D<sub>2</sub>]] |
|||
| 3 |
|||
| |
|||
|- |
|||
| [[多巴胺D3受体|D<sub>3</sub>]] |
|||
| 6.3 |
|||
| |
|||
|- |
|||
| [[σ1受体|σ<sub>1</sub>]] |
|||
| ? |
|||
| |
|||
|- |
|||
| [[肾上腺素α1A受体|α<sub>1A</sub>]] |
|||
| 1.3 |
|||
| |
|||
|- |
|||
| [[肾上腺素α2A受体|α<sub>2A</sub>]] |
|||
| 2.1 |
|||
| |
|||
|- |
|||
| [[TAAR1|TAAR<sub>1</sub>]] |
|||
| 2.2 |
|||
| |
|||
|- |
|||
| [[组胺H1受体|H<sub>1</sub>]] |
|||
| 0.22 |
|||
| |
|||
|- |
|||
| [[咪唑啉1受体|I<sub>1</sub>]] |
|||
| ? |
|||
| |
|||
|- |
|||
| [[血清素转运体|SERT]] |
|||
| 6 |
|||
| |
|||
|- |
|||
| [[多巴胺转运体|DAT]] |
|||
| 22 |
|||
| |
|||
|- |
|||
| [[去甲肾上腺素转运体|NET]] |
|||
| 6.5 |
|||
| |
|||
|} |
|||
== 法律地位 == |
== 法律地位 == |
||
| 第116行: | 第55行: | ||
{{中华人民共和国管制药品}} |
{{中华人民共和国管制药品}} |
||
[[Category:中华人民共和国管制药品]] |
|||
2025年3月8日 (六) 23:30的最新版本
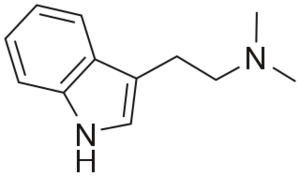 | |
 | |
| 化学数据 | |
|---|---|
| 化学式 | C12H16N2 |
| 摩尔质量 | 188.27 g·mol−1 |
| 识别信息 | |
| IUPAC名称 | 2-(1H-吲哚-3-基)-N,N-二甲基乙胺 |
| CAS号 | |
| PubChem CID | |
| 药理数据[1] | |
| 5HT1A(↑) | |
| 5HT1B | |
| 5HT1D | |
| 5HT2A(↑) | |
| 5HT2B | |
| 5HT2C(↑) | |
| HRH1 | |
| DRD1 | |
| DRD2 | |
| DRD3 | |
| SERT(↓) | |
| DAT | |
| NET | |
| ADRA1A | |
| ADRA2A | |
| TAAR1 | |
| I1 | |
N,N-二甲基色胺(N,N-Dimethyltryptamine,DMT)是一种色胺类致幻剂、诱导剂。它存在于包括人类在内的许多动物和植物中。
药理学
DMT以低于0.6μmol/L的亲和力非选择性地与以下血清素受体结合:5-HT1A、[2][3][4] 5-HT1B、[2][5] 5-HT1D、[2][4][5] 5-HT2A、 [2][4][5][6] 5-HT2B、[2][5] 5-HT2C、[2][5][6] 5-HT6、[2][5]5-HT7。[2][5]它在5-HT1A、[3]5-HT2A和5-HT2C上作为激动剂起效。[2][5][6]它对其他血清素受体的活性仍有待确定。特别令人感兴趣的是确定其对人类5-HT2B受体的功效,因为两项体外测定证明了DMT对该受体具有0.108 µmol/L、[5]0.184 µmol/L[2]的高亲和力。长期或频繁使用对5-HT2B受体表现出优先高亲和力和明显激动作用的血清素药物与瓣膜性心脏病存在因果关系。[7][8][9]它还被证明对多巴胺D1、肾上腺素能α1、肾上腺素α2、咪唑啉-1和σ1受体具有亲和力。[4][5][10] 多个证据证明σ1受体在浓度为50~100 μmol/L时被激活,[11]而在其他受体结合位点的效力尚不清楚。它还在体外被证明是人类血小板中表达的细胞表面血清素转运蛋白(SERT)的底物、大鼠囊泡单胺转运蛋白2(VMAT2)、 秋粘虫的Sf9细胞中瞬时表达。DMT在血小板抑制SERT回收血清素的平均浓度为4±0.70 µmol/L,抑制VMAT2血清素回收的平均浓度为93±6.8 µmol/L。[12]与其他所谓的“经典致幻剂”一样,[13]DMT的致幻作用的很大一部分可归因于5-HT2A受体的功能选择性激活。[14][2][15][16][17][18][19]DMT浓度在体外对人类5-HT2A受体产生50%的最大效应(EC50)的范围为0.118~0.983 µmol/L,[2][5][6][20] 该值范围与给予完全迷幻剂量后在血液和血浆中测量的浓度范围非常吻合。由于DMT已被证明对人类血清素2C受体的功效 (EC50) 略高于对2A受体的功效,[5][6] 因此5-HT2C也可能对DMT的整体效果有影响。[16][21]其他受体例如5-HT1A[4][16][18]、σ1[11][22]等也可能发挥作用。
法律地位
中国
DMT在中国于2013年被列入精神药品品种目录第一类。
引用文献
- ↑ Rickli A, Moning OD, Hoener MC, Liechti ME (August 2016). "Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens" (PDF). European Neuropsychopharmacology. 26 (8): 1327–1337. doi:10.1016/j.euroneuro.2016.05.001. PMID 27216487. S2CID 6685927.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 Keiser MJ, Setola V, Irwin JJ, Laggner C, Abbas AI, Hufeisen SJ, Jensen NH, Kuijer MB, Matos RC, Tran TB, Whaley R, Glennon RA, Hert J, Thomas KL, Edwards DD, Shoichet BK, Roth BL (November 2009). "Predicting new molecular targets for known drugs". Nature. 462 (7270): 175–181. Bibcode:2009Natur.462..175K. doi:10.1038/nature08506. PMC 2784146. PMID 19881490.
- ↑ 3.0 3.1 Deliganis AV, Pierce PA, Peroutka SJ (June 1991). "Differential interactions of dimethyltryptamine (DMT) with 5-HT1A and 5-HT2 receptors". Biochemical Pharmacology. 41 (11): 1739–1744. doi:10.1016/0006-2952(91)90178-8. PMID 1828347.
- ↑ 4.0 4.1 4.2 4.3 4.4 Pierce PA, Peroutka SJ (1989). "Hallucinogenic drug interactions with neurotransmitter receptor binding sites in human cortex". Psychopharmacology. 97 (1): 118–122. doi:10.1007/BF00443425. PMID 2540505. S2CID 32936434.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 Ray TS (February 2010). "Psychedelics and the human receptorome". PLOS ONE. 5 (2): e9019. Bibcode:2010PLoSO...5.9019R. doi:10.1371/journal.pone.0009019. PMC 2814854. PMID 20126400.
- ↑ 6.0 6.1 6.2 6.3 6.4 Smith RL, Canton H, Barrett RJ, Sanders-Bush E (November 1998). "Agonist properties of N,N-dimethyltryptamine at serotonin 5-HT2A and 5-HT2C receptors" (PDF). Pharmacology, Biochemistry, and Behavior. 61 (3): 323–330. doi:10.1016/S0091-3057(98)00110-5. PMID 9768567. S2CID 27591297.[永久失效链接]
- ↑ Rothman RB, Baumann MH (May 2009). "Serotonergic drugs and valvular heart disease". Expert Opinion on Drug Safety. 8 (3): 317–329. doi:10.1517/14740330902931524. PMC 2695569. PMID 19505264.
- ↑ Roth BL (January 2007). "Drugs and valvular heart disease". The New England Journal of Medicine. 356 (1): 6–9. doi:10.1056/NEJMp068265. PMID 17202450.
- ↑ Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB (January 2007). "Functional selectivity and classical concepts of quantitative pharmacology". The Journal of Pharmacology and Experimental Therapeutics. 320 (1): 1–13. doi:10.1124/jpet.106.104463. PMID 16803859. S2CID 447937.
- ↑ Burchett SA, Hicks TP (8月 2006). "The mysterious trace amines: protean neuromodulators of synaptic transmission in mammalian brain" (PDF). Progress in Neurobiology. 79 (5–6): 223–246. doi:10.1016/j.pneurobio.2006.07.003. OCLC 231983957. PMID 16962229. S2CID 10272684. Archived from the original (PDF) on 1 2月 2012.
{{cite journal}}:|archive-date=/|archive-url=timestamp mismatch; 1 2月 2012 suggested (help) - ↑ 11.0 11.1 Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE (February 2009). "The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator". Science. 323 (5916): 934–937. Bibcode:2009Sci...323..934F. doi:10.1126/science.1166127. PMC 2947205. PMID 19213917.
- ↑ Cozzi NV, Gopalakrishnan A, Anderson LL, Feih JT, Shulgin AT, Daley PF, Ruoho AE (December 2009). "Dimethyltryptamine and other hallucinogenic tryptamines exhibit substrate behavior at the serotonin uptake transporter and the vesicle monoamine transporter" (PDF). Journal of Neural Transmission. 116 (12): 1591–1599. doi:10.1007/s00702-009-0308-8. PMID 19756361. S2CID 15928043. Archived from the original (PDF) on 17 June 2010. Retrieved 20 November 2010.
- ↑ Glennon RA (1994). "Classical hallucinogens: an introductory overview" (PDF). In Lin GC, Glennon RA (eds.). Hallucinogens: An Update. NIDA Research Monograph Series. Vol. 146. Rockville, MD: U.S. Dept. of Health and Human Services, Public Health Service, National Institutes of Health, National Institute on Drug Abuse. p. 4. Archived (PDF) from the original on 2011-07-25.[永久失效链接]
- ↑ Strassman RJ, Qualls CR (February 1994). "Dose-response study of N,N-dimethyltryptamine in humans. I. Neuroendocrine, autonomic, and cardiovascular effects". Archives of General Psychiatry. 51 (2): 85–97. doi:10.1001/archpsyc.1994.03950020009001. PMID 8297216.
- ↑ Fantegrossi WE, Murnane KS, Reissig CJ (January 2008). "The behavioral pharmacology of hallucinogens". Biochemical Pharmacology. 75 (1): 17–33. doi:10.1016/j.bcp.2007.07.018. PMC 2247373. PMID 17977517.
- ↑ 16.0 16.1 16.2 Nichols DE (February 2004). "Hallucinogens". Pharmacology & Therapeutics. 101 (2): 131–181. doi:10.1016/j.pharmthera.2003.11.002. PMID 14761703.
- ↑ Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, Vogel H, Hell D (December 1998). "Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action". NeuroReport. 9 (17): 3897–3902. doi:10.1097/00001756-199812010-00024. PMID 9875725. S2CID 37706068.
- ↑ 18.0 18.1 Strassman RJ (1996). "Human psychopharmacology of N,N-dimethyltryptamine" (PDF). Behavioural Brain Research. 73 (1–2): 121–124. doi:10.1016/0166-4328(96)00081-2. PMID 8788488. S2CID 4047951.[永久失效链接]
- ↑ Glennon RA, Titeler M, McKenney JD (December 1984). "Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents". Life Sciences. 35 (25): 2505–2511. doi:10.1016/0024-3205(84)90436-3. PMID 6513725.
- ↑ Roth BL, Choudhary MS, Khan N, Uluer AZ (February 1997). "High-affinity agonist binding is not sufficient for agonist efficacy at 5-hydroxytryptamine2A receptors: evidence in favor of a modified ternary complex model" (PDF). The Journal of Pharmacology and Experimental Therapeutics. 280 (2): 576–83. PMID 9023266.
- ↑ Canal CE, Olaghere da Silva UB, Gresch PJ, Watt EE, Sanders-Bush E, Airey DC (April 2010). "The serotonin 2C receptor potently modulates the head-twitch response in mice induced by a phenethylamine hallucinogen". Psychopharmacology. 209 (2): 163–174. doi:10.1007/s00213-010-1784-0. PMC 2868321. PMID 20165943.
- ↑ Su TP, Hayashi T, Vaupel DB (March 2009). "When the endogenous hallucinogenic trace amine N,N-dimethyltryptamine meets the sigma-1 receptor". Science Signaling. 2 (61): pe12. doi:10.1126/scisignal.261pe12. PMC 3155724. PMID 19278957.
外部引用
此条目使用了来自外部的条目,它们可能需要在站内被创建或编辑
来自wikipedia的条目: